* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 14
Survey
Document related concepts
Transcript
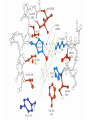
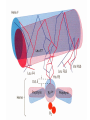
Protein Function Globins and Antibodies 3/12/2003 Quaternary structure of deoxy- and oxyhemoglobin T-state R-state D-2,3-bisphosphoglycerate (BPG) O O C H C OPO3-- H C OPO3-- BPD binds to hemoglobin and decreases the oxygen affinity and keeps it in the deoxy form. H BPG binds 1:1 with a K=1x10-5 M to the deoxy form but weakly to the oxy form BPG binds specifically to the deoxy state and stabilizes it in the T state. In the R state the central cavity is too narrow for BPG to fit or bind. The P50 value of stripped hemoglobin increases from 12 to 22 torr by 4.7 mM BPG At 100 torr or arterial blood, hemoglobin is 95% saturated At 30 torr or venous blood, hemoglobin is 55% saturated Hemoglobin releases 40% of its oxygen. In the absence of BPG, little oxygen is released. Between BPG, CO2, H+, and Cl- all O2 binding is accounted for. BPG levels are partially responsible for High-Altitude adaptation BPG restores the 37% release of O2 at higher elevations between arterial and venous blood Hemoglobin mutants There are about 500 variants of hemoglobin 95% are single amino acid substitution. 5 % of the worlds population carries a different sequence form the normal. •Changes in surface charge •Changes in internally located residues •Changes stabilizing Methemoglobin •Changes in the a1-b2 contact Changes in surface rarely change the function of hemoglobin with the exception of the sickle cell mutation. Internal residues cause the hemoglobin to contort to different shapes and alter its binding properties. Heinz bodies are precipitated aggregates of hemoglobin. Usually cause hemolytic anemia characteristic by cell lysis. Hb Hammersmith Phe CD1(42)b Ser. The Phe wedges the heme in place, without it the heme falls out of the protein. Hb Bristol Val E11(67)b Asp occludes O2 from the pocket. Hb Bibba substitutes a Pro in the middle of H helix kinks the chain. Hb Savannah replaces Gly B6(24)b Val where the B helix crosses the E helix. Stabilizing Methemoglobin. Or the Fe(III) state eliminates the oxygen binding to the heme. Diagnostic of a blue color or cyanosis. Hb Boston His E7(58)a Tyr (the distal His) Hb Milwaukee Val E11(67)b Glu Hb Iwate His F8(87)a Tyr “black mouth disease Japan They have chocolate brown blood. Changes at the a1-b2 interface usually have lower Hill coefficients. Stabilize either the T or R state Polycythemia, a ruddy complexion Hb Yakima Asp G1(99)b His eliminates H-bonding that stabilizes the T form at the a1-b2 interface and stabilizes the R state P50 =12 torr Hb Kansas Asn G4(102)b Thr eliminates H-bonding that stabilizes the T form at the a1-b2 interface and so when O2 binds it remains in the T state P50 =70 torr Fetal Hemoglobin •Fetal hemoglobin has a different b subunit called a g subunit or a2g2. •In Fetal hemoglobin, BPG does not affect this variant and the baby’s blood will get its oxygen from the mothers hemoglobin. •The transfer of oxygen is from the mother (less tightly bond) to the baby (more tightly bond). Antibodies and the immune response