* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pathological forms of hemoglobin. Acid
Photosynthesis wikipedia , lookup
Cell theory wikipedia , lookup
Developmental biology wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
High-altitude adaptation in humans wikipedia , lookup
Regeneration in humans wikipedia , lookup
Homeostasis wikipedia , lookup
Biochemistry wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Organisms at high altitude wikipedia , lookup
Human genetic resistance to malaria wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
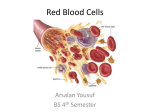
Pathological forms of hemoglobin. Acidbase state of blood. Hemoglobin Hemoglobin (also spelled haemoglobin and abbreviated Hb or Hgb) is the iron-containing oxygen-transport metalloprotein in the red blood cells of vertebrates,and the tissues of some invertebrates. In mammals, the protein makes up about 97% of the red blood cell's dry content, and around 35% of the total content (including water). Hemoglobin transports oxygen from the lungs or gills to the rest of the body (i.e. the tissues) where it releases the oxygen for cell use. Hemoglobin has an oxygen binding capacity of between 1.36 and 1.37 ml O2 per gram of hemoglobin, which increases the total blood oxygen capacity seventyfold. Hemoglobin is also found in outside red blood cells and their progenitor lines. Other cells that contain hemoglobin include the A9 dopaminergic neurons in the substantia nigra, macrophages, alveolar cells, and mesangial cells in the kidney. In these tissues, hemoglobin has a non-oxygen carrying function as an antioxidant and a regulator of iron metabolism. Methemoglobin • The iron ion may either be in the Fe2+ or Fe3+ state, but ferrihemoglobin (methemoglobin) (Fe3+) cannot bind oxygen. In binding, oxygen temporarily oxidizes (Fe2+) to (Fe3+), so iron must exist in the +2 oxidation state to bind oxygen. The enzyme methemoglobin reductase reactivates hemoglobin found in the inactive (Fe3+) state by reducing the iron center. Carboxyhemoglobin • The binding of oxygen is affected by molecules such as carbon monoxide (CO) (for example from tobacco smoking, car exhaust and incomplete combustion in furnaces). CO competes with oxygen at the heme binding site. Hemoglobin binding affinity for CO is 200 times greater than its affinity for oxygen, meaning that small amounts of CO dramatically reduce hemoglobin's ability to transport oxygen. When hemoglobin combines with CO, it forms a very bright red compound called carboxyhemoglobin, which may cause the skin of CO poisoning victims to appear pink in death, instead of white or blue. Acid-base homeostasis • Acid-base homeostasis is the part of human homeostasis concerning the proper balance between acids and bases, in other words, the pH. The body is very sensitive to its pH level, so strong mechanisms exist to maintain it. Outside the acceptable range of pH, proteins are denatured and digested, enzymes lose their ability to function, and death may occur. Mechanism • • • The body's acid-base balance is tightly regulated. Several buffering agents that reversibly bind hydrogen ions and impede any change in pH exist. Extracellular buffers include bicarbonate and ammonia, whereas proteins and phosphate act as intracellular buffers. The bicarbonate buffering system is especially key, as carbon dioxide (CO2) can be shifted through carbonic acid (H2CO3) to hydrogen ions and bicarbonate (HCO3-) as shown below. Acid-base imbalances that overcome the buffer system can be compensated in the short term by changing the rate of ventilation. This alters the concentration of carbon dioxide in the blood, shifting the above reaction according to Le Chatelier's principle, which in turn alters the pH. For instance, if the blood pH drops too low (acidemia), the body will compensate by increasing breathing, expelling CO2, and shifting the above reaction to the right such that less hydrogen ions are free; thus the pH will rise back to normal. For alkalemia, the opposite occurs. The kidneys are slower to compensate, but renal physiology has several powerful mechanisms to control pH by the excretion of excess acid or base. In responses to acidosis, tubular cells reabsorb more bicarbonate from the tubular fluid, collecting duct cells secrete more hydrogen and generate more bicarbonate, and leads to increased formation of the NH3 buffer. In responses to alkalosis, the kidney may excrete more bicarbonate by decreasing hydrogen ion secretion from the tubular epithelial cells, and increasing rates of glutamine metabolism and ammonia excretion.