* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download chapter 4 -aromatic compounds
Hydroformylation wikipedia , lookup
Volatile organic compound wikipedia , lookup
George S. Hammond wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Homoaromaticity wikipedia , lookup
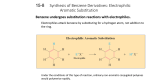
ORGANIC CHEMISTRY CHM 207 CHAPTER 4: AROMATIC COMPOUNDS (BENZENE AND TOLUENE) NOR AKMALAZURA JANI Aromatic compounds • Organic compound that contains a benzene ring in its molecule is known as an aromatic compounds. • Sometimes called arenes. • Molecular formula: C6H6 • Represented as a regular hexagon containing an inscribed circle. Structure of Benzene • Can be represented in two abbreviated ways. • The corner of each hexagon represents a carbon and a hydrogen atom. Kekulé Structure of Benzene Molecular formula is C6H6 All the hydrogen atoms are equivalent Each carbon atom must have four covalent bonds. Resonance Structure • Resonance theory: the structure of benzene is a resonance hybrid structure of two Kekulé cononical forms. • The hybrid structure is often represented by a hexagon containing an inscribed circle. represents a resonance hybrid between the two • Hexagonal ring – 6 carbon-carbon bonds are equal. • Circle – delocalised electrons of the benzene ring CRITERIA OF AROMATIC COMPOUNDS • Structure must be cyclic, containing some number of conjugated pi bonds. • Each atom in the ring must have an unhybridized p orbital. (The ring atoms are usually sp2 hybridized or occasionally sp hybridized). • The unhybridized p orbitals must overlap to form a continuous ring of parallel orbitals. The structure must be planar (or nearly planar) for effective overlap to occur. • Delocalization of the pi electrons over the ring must lower the electronic energy. * Antiaromatic compound: fulfills the first three criteria, but delocalization of the pi electrons over the ring increase the electronic energy. Huckel’s rule • Used to determine aromaticity for planar, cyclic organic compounds with a continous ring of overlapping porbitals. • If the number of pi (π) electrons in the monocyclic system is (4N+2), the system is aromatic. N is 0, 1, 2, 3….. • Systems that have 2, 6 and 10 pi electrons for N = 0, 1, 2 is a aromatic. • Systems that have 4, 8, and 12 pi electrons for N = 1, 2, 3 are antiaromatic. Naming Aromatic Compounds • A substituted benzene is derived by replacing one or more of benzene’s hydrogen atoms with an atom or group of atoms. • A monosubstituted benzene has the formula C6H5G where G is the group that replaces a hydrogen atom. • All hydrogens in benzene are equivalent. • It does not matter which hydrogen is replaced by G. Monosubstituted Benzenes • Some monosubstituted benzenes are named by adding the name of the substituent group as a prefix to the word benzene. • The name is written as one word. nitro group nitrobenzene ethyl group ethylbenzene • Certain monosubstituted benzenes have special names. • These are parent names for further substituted compounds. hydroxy group methyl group toluene phenol carboxyl group amino group benzoic acid aniline Disubstituted Benzenes • Three isomers are possible when two substituents replace hydrogen in a benzene molecule. • The prefixes ortho-, meta- and para- (o-, m- and p-) are used to name these disubstituted benzenes. ortho disubstituted benzene substituents on adjacent carbons ortho-dichlorobenzene (1,2-dichlorobenzene) mp –17.2oC, bp 180.4oC meta disubstituted benzene substituents on adjacent carbons meta-dichlorobenzene (1,3-dichlorobenzene) mp –24.82oC, bp 172oC para disubstituted benzene substituents are on opposite sides of the benzene ring para-dichlorobenzene (1,4-dichlorobenzene) mp 53.1, bp 174.4oC When one substituent corresponds to a monosubstituted benzene with a special name, the monosubstituted compound becomes the parent name for the disubstituted compound. phenol 3-nitrophenol When one substituent corresponds to a monosubstituted benzene with a special name, the monosubstituted compound becomes the parent name for the disubstituted compound. toluene 3-nitrotoluene Tri- and Polysubstituted Benzenes • When a benzene ring has three or more substituents, the carbon atoms in the ring are numbered. • Numbering starts at one of the substituent groups. • The numbering direction can be clockwise or counterclockwise. • Numbering must be in the direction that gives the substituent groups the lowest numbers. 6-chloro clockwise numbering 1-chloro 6 4-chloro 5 1 4 2 3 1,4,6-trichlorobenzene counterclockwise numbering chlorine substituents have lower numbers 4-chloro 2-chloro 1-chloro 2 3 1 4 6 5 1,2,4-trichlorobenzene • When a compound is named as a derivative of the special parent compound, the substituent of the parent compound is considered to be C-1 of the ring. 1 1 2 6 6 2 5 3 4 3 5 4 toluene 2,4,6trinitrotoluene (TNT) • When the hydrocarbon chain attached to the benzene ring is small, the compound is named as benzene derivative. • Example: CH2CH3 ethylbenzene Naming compounds that cannot be easily named as benzene derivatives Benzene named as a substituent on a molecule with another functional group as its root by the prefix phenyl. diphenylmethane 4-phenyl-2-pentene The phenyl group, C6H5CH2 phenyl CH=CH2 common name phenylethene benzyl NH2 CH2Cl phenylamine benzyl chloride • If the hydrocarbon chain contains more than three carbon atoms, phenyl is used as part of the name. • Examples: CH3 CH2(CH2)5CH3 1-phenylheptane C CH2 CH3 Br 2-bromo-2-phenylbutane PHYSICAL PROPERTIES OF BENZENE AND ITS DERIVATIVES • Benzene derivatives tend to be more symmetrical than similar aliphatic compounds, and pack better into crystals and have higher melting points. • Density: - Slightly dense than non-aromatic analogues, but still less dense than water. - halogenated benzenes are denser than water. • Insoluble in water • Boiling points depends on the dipole moments of compounds. REACTION OF BENZENE ELECTROPHILIC SUBSTITUTION REACTIONS OF BENZENE stability of π-electron system is lost when benzene undergoes addition reactions. benzene and its derivatives undergo substitution reaction rather than addition reactions. product of substitution reactions: aromatic compounds and not saturated compounds. Mechanism of electrophilic substitution of benzene Step 1: Electrophilic addition of the benzene ring H + E E slow arenium ion (a carbocation) Step 2: Deprotonation of the arenium ion H E E Nu- fast nucleophile H Nu ELECTROPHILIC SUBSTITUTION REACTIONS a) Halogenation X H X2 H2SO4 or FeX3 HX halobenzene b) Nitration NO2 H HNO3 H2SO4 2H2O nitrobenzene c) Sulphonation SO3H H SO3 H2SO4 benzenesulphonic acid ELECTROPHILIC SUBSTITUTION REACTIONS d) Friedel-Crafts alkylation H CH3 AlCl3 HCl CH3Cl toluene e) Friedel-Crafts acylation H O AlCl3 O C CH3 HCl CH3CCl acetophenone Reagents, electrophiles and catalysts in electrophilic substitution reactions Reactions Reagents Catalysts Electrophiles Halogenation Cl2 or Br2 AlCl3, AlBr3, FeCl3 or FeBr3 Cl , Br Nitration HNO3 H2SO4 NO2 Alkylation RCl AlCl3 R RCH=CH2 H2SO4 RCH-CH3 RCOCl AlCl3 Acylation RCO Sulphonation SO3 H2SO4 SO3H HALOGENATION OF BENZENE a)Chlorination Cl Cl2 AlCl3 HCl chlorobenzene b)Bromination Br Br2 FeBr3 HBr bromobenzene c) Iodination I 1/2I2 NO2 HNO3 iodobenzene H2O MECHANISM: BROMINATION OF BENZENE Step 1: Formation of a stronger electrophile Br Br Br Br FeBr3 FeBr3 Br2.FeBr3 intermediate (a stronger electrophile than Br2) Step 2: Electrophilic attack and formation of the sigma complex H H H H H Br H Br Br H H H H H Br H H H H H H H H H FeBr4- Step 3: Loss of a proton gives the products H Br H H H FeBr4 - H H Br HBr H H H H H sigma complex H H FeBr3 H H Br FeBr3 MECHANISM: NITRATION OF BENZENE Step 1: Formation of the nitronium ion, NO2+ HO SO3 H H2O + NO2+ + HSO4- HO NO2 Step 2: Formation of an arenium ion as a result of electrophilic addition H NO2 NO2+ slow nironium ion arenium ion Step 3: Loss of a proton gives the products H NO2 HSO4- NO2 fast H2SO4 MECHANISM: FRIEDEL-CRAFTS ALKYLATION Step 1: Formation of electrophile CH3 H C Cl CH3 AlCl3 CH3 H C CH3 - AlCl4 carbocation (electrophile) Step 2: Formation of an arenium ion H C CH3 CH3 H CH(CH3)2 arenium ion Step 3: Loss of a proton H CH(CH3)2 CH(CH3)2 - AlCl4 HCl + AlCl3 MECHANISM: FRIEDEL-CRAFTS ACYLATION Step 1: Formation of electrophile O O CH3 C Cl - AlCl4 CH3 C AlCl3 Step 2: Formation of an arenium ion O H C O CH3 CH3 C Step 3: Loss of a proton O H C AlCl4- O CH3 C CH3 HCl + AlCl3 Ortho-Para and Meta Directing Substituents • When substituted benzenes undergo further substituents, the substituent group present in the benzene derivative will influence electrophilic substitution in 2 ways which are: i) Reactivity ii)Orientation EFFECTS OF SUBSTITUENTS ON THE REACTIVITY OF ELECTROPHILIC AROMATIC SUBSTITUTION • Substituent group present in the benzene ring can influence the rate of reaction of further substitutions. • Electron-donating groups make the ring more reactive (called activating groups) thus influence the reaction become faster. • Electron-withdrawing groups make the ring less reactive (called deactivating groups) thus influence the reaction become slower. EFFECTS OF SUBSTITUENTS ON THE ORIENTATION OF ELECTROPHILIC AROMATIC SUBSTITUTION • A substituents group already in the ring influences the position of further electrophilic substitution whether at ortho, meta or para position. • Ortho-para directors: the groups that tend to direct electrophilic substitution to the C2 and C4 positions. • Meta directors: the groups that tend to direct electrophilic substitution to the C3 position. Effetcs of substituent groups on the benzene ring Activating groups (electron donating) -NH2 -OH -OR -NHCOCH3 -R Deactivating groups (electron-withdrawing) -F -Cl -Br -I O C O C R OH ortho-para directors ortho-para directors C N NO2 O C SO3H OR NR3 meta directors Example: CH2CH3 Br2 CH2CH3 Br CH2CH3 CH2CH3 Br FeBr3 Br -CH2CH3 = ortho and para directors ortho position para position major products meta position minor product Example: NO2 NO2 NO2 NO2 Br Br2 FeBr3 -NO2 = meta director Br meta position Br ortho position para position major product minor products REACTIONS OF BENZENE DERIVATIVES • Alkylbenzene such as toluene (methylbenzene) resembles benzene in many of its chemical properties. • It is preferable to use toluene because it is less toxic. • The methyl group activates the benzene nucleus. • Toluene reacts faster than benzene in all electrophilic substitutions. Reactions of toluene Reactions of the methyl group Substitution -halogenation Oxidation Reactions of the benzene ring Electrophilic substitutions - Halogenation - Nitration - Friedel-Crafts reactions - Sulfonation Addition reaction -hydrogenation SIDE-CHAIN REACTIONS OXIDATION REACTION OF ALKYLBENZENE O + CH2 R hot, conc., KMnO4/H C OH reflux examples: CH3 O + hot, conc., KMnO4/H C OH reflux + CH2 CH3 hot, conc., KMnO4/H reflux CH3 CH3 hot, conc., KMnO4/H+ reflux O C OH COOH COOH HALOGENATION OF TOLUENE Side chain substitution CH3 CH2 Cl Cl2 uv light HCl (chloromethyl)benzene CHCl2 CH2 Cl Cl2 uv light HCl (dichloromethyl)benzene CCl3 CHCl2 Cl2 uv light HCl (trichloromethyl)benzene * Bromination of toluene takes place under similar conditions to yield corresponding bromine derivatives. SYNTHESIZING A SUBSTITUTED AROMATIC COMPOUNDS Synthesis m-chloronitrobenzene starting from benzene NO2 ? Cl • Two substituents: -NO2 (meta-directing) and –Cl (orthoand para-directing) • Cannot nitrate chlorobenzene because the wrong isomer (o- and p-chloronitrobenzenes) would formed. Cl chlorobenzene HNO3, H2SO4 NO2 NO2 Cl Cl2, FeCl3 m-chloronitrobenzene nitrobenzene TWO STEPS: benzene NO2 NO2 HNO3 Cl2 H2SO4 FeCl3 nitrobenzene Cl m-chloronitrobenzene SYNTHESIZING A SUBSTITUTED AROMATIC COMPOUNDS Synthesis p-bromobenzoic acid starting from benzene COOH ? Br • Two substituents: -COOH (meta-directing) and –Br (ortho- and paradirecting) • Cannot brominated benzioc acid because the wrong isomer (m-bromobenzoic acid) would formed. • Oxidation of alkylbenzene side chains yields benzoic acids. • Intermediate precursor is p-bromotoluene CH3 COOH KMnO4 Br Br Immediate precursor of p-bromotoluene: i) Bromination of toluene or ii) Methylation of bromobenzene CH3 CH3 Br2 FeCl3 Br or Br separate the isomer CH3 CH3Cl Br CH3 AlCl3 Br CH3 Br separate the isomer Immediate precursor of toluene: i) Benzene was methylated in a Friedel-Crafts reaction CH3 CH3Cl AlCl3 benzene toluene Immediate precursor of bromobenzene: i) Bromination of benzene Br2 FeBr3 benzene Br bromobenzene TWO WORKABLE ROUTES FROM BENZENE TO p-BROMOBENZOIC ACID Br2 FeBr3 CH3Cl Br AlCl3 CH3 benzene CH3Cl AlCl3 CH3 Br2 FeBr3 Br COOH KMnO4 Br USES OF BENZENE AND TOLUENE • Benzene: - as solvent for oils and fats - starting material for making other chemicals. For example, benzene is used in the cumene process to produce phenol. - making organic compounds such as phenylethene (styrene) and nitrobenzene. These organic compounds are then used to make plastics (polystyrene), dyes and nylon. USES OF BENZENE AND TOLUENE • Toluene: - A common solvent, able to dissolve paints, paint thinners, silicone sealants, many chemical reactants, rubber, printing ink, adhesives (glues), lacquers, leather tanners and disinfectants. - As a solvent to create a solution of carbon nanotubes. - Dealkylation to benzene (industrial uses). - As an octane booster in gasoline fuels used in internal combustion engines. -As a coolant in nuclear reactor system loops.