* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chapter 19 (part 1) - Nevada Agricultural Experiment
Survey
Document related concepts
Transcript
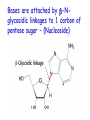
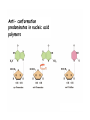
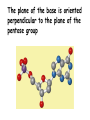
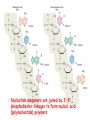
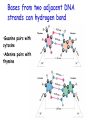
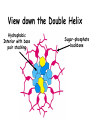
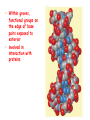
Chapter 19 (part 1) Nucleic Acids Nucleic Acids Are Essential For Information Transfer in Cells • Information encoded in a DNA molecule is transcribed via synthesis of an RNA molecule • The sequence of the RNA molecule is "read" and is translated into the sequence of amino acids in a protein. Central Dogma of Biology Nucleic Acids • First discovered in 1869 by Miescher. • Found as a precipitate that formed when extracts from nuclei were treated with acid. • Compound contained C, N, O, and high amount of P. • Was an acid compound found in nuclei therefore named nucleic acid Nucleic Acids • 1944 Oswald, Avery, MacLeod and McCarty demonstrated that DNA is the molecule that carrier genetic information. • 1953 Watson and Crick proposed the double helix model for the structure of DNA Nucleic Acids • Nucleic acids are long polymers of nucleotides. • Nucleotides contain a 5 carbon sugar, a weakly basic nitrogenous compound (base), one or more phosphate groups. • Nucleosides are similar to nucleotides but have no phosphate groups. Pentoses of Nucleotides • D-ribose (in RNA) • 2-deoxy-D-ribose (in DNA) • The difference - 2'OH vs 2'-H • This difference affects secondary structure and stability Nitrogenous Bases Bases are attached by b-Nglycosidic linkages to 1 carbon of pentose sugar – (Nucleoside) Nucleosides • Base is linked via a b-Nglycosidic bond • The carbon of the glycosidic bond is anomeric • Named by adding -idine to the root name of a pyrimidine or -osine to the root name of a purine • Conformation can be syn or anti • Sugars make nucleosides more water-soluble than free bases Anti- conformation predominates in nucleic acid polymers Nucleotides • Phosphate ester of nucleosides The plane of the base is oriented perpendicular to the plane of the pentose group Other Functions of Nucleotides • Nucleoside 5'-triphosphates are carriers of energy • Bases serve as recognition units • Cyclic nucleotides are signal molecules and regulators of cellular metabolism and reproduction • ATP is central to energy metabolism • GTP drives protein synthesis • CTP drives lipid synthesis • UTP drives carbohydrate metabolism • Nucleotide monomers are joined by 3’-5’ phosphodiester linkages to form nucleic acid (polynucleotide) polymers Nucleic Acids • Nucleic acid backbone takes on extended conformation. • Nucleotide residues are all oriented in the same direction (5’ to 3’) giving the polymer directionality. • The sequence of DNA molecules is always read in the 5’ to 3’ direction Bases from two adjacent DNA strands can hydrogen bond •Guanine pairs with cytosine •Adenine pairs with thymine Base pairing evident in DNA compositions H-bonding of adjacent antiparallel DNA strands form double helix structure Properties of DNA Double Helix • Distance between the 2 sugar-phosphate backbones is always the same, give DNA molecule a regular shape. • Plane of bases are oriented perpendicular to backbone • Hydrophillic sugar phosphate backbone winds around outside of helix • Noncovalent interactions between upper and lower surfaces of base-pairs (stacking) forms a closely packed hydrophobic interior. • Hydrophobic environment makes H-bonding between bases stronger (no competition with water) • Cause the sugar-phosphate backbone to twist. View down the Double Helix Hydrophobic Interior with base pair stacking Sugar-phosphate backbone Structure of DNA Double Helix • Right handed helix • Rise = 0.33 nm/nucleotide • Pitch = 3.4 nm / turn • 10.4 nucleotides per turn • Two groves – major and minor • Within groves, functional groups on the edge of base pairs exposed to exterior • involved in interaction with proteins. Factors stabilizing DNA double Helix • Hydrophobic interactions – burying hydrophobic purine and pyrimidine rings in interior • Stacking interactions – van der Waals interactions between stacked bases. • Hydrogen Bonding – H-bonding between bases • Charge-Charge Interactions – Electrostatic repulsions of negatively charged phosphate groups are minimized by interaction with cations (e.g. Mg2+)