* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry - Ms. Chambers' Biology
Microbial metabolism wikipedia , lookup
Biosynthesis wikipedia , lookup
Photosynthesis wikipedia , lookup
Carbon sink wikipedia , lookup
Isotopic labeling wikipedia , lookup
Proteolysis wikipedia , lookup
Metalloprotein wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
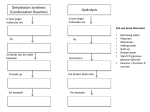
Carbon compounds Biochemistry A crossover field of chemistry Biochemists have to understand both the living world and the chemical world Every living thing uses the same basic chemical compounds to live their lives. We are talking smaller than cells... Carbon based molecules are basis of life! Why Carbon? Carbon has 4 valence shell electrons 6 C Carbon 12.011 Why is that important? Each of carbon's valence shell electrons can bond with another atom And carbon can even bond with itself Carbon can form rings, chains, and other shapes of atoms Carbon literally forms the backbone of biology! NO LIFE has been observed that is not carbon based. Carbon atoms love to bond, and accordingly, very LONG and COMPLEX molecules can result from that bonding! Warmup 9/20 What do the following prefixes mean? Macro Micro Poly Mono- Play-doh Macromolecules“giant molecules” Formed by polymerization When large compounds are built by joining smaller molecules together (legos) Carbohydrates Lipids There are FOUR groups of Macromolecules Nucleic Acids Proteins Carbohydrates Have CHO carbon, hydrogen and oxygen atoms In a 1:2:1 ratio Main source of energy for living things Examples: Sugars- (monosaccharides –not all) Glucose (C6H12O6 ) Starches (polysaccharides) Glycogen, cellulose Sugars: Short-chain carbs Monosaccharides- monomers: mono= “single” meros (Gk) = part Examples: glucose fructose galactose “-ose” denotes sugars The following are considered “simple sugars”- monosaccharides: Sucrose If you add two monosaccharides you get a Disacchiride mono (1)+ mono (1) di (2) Glucose + fructose sucrose Starches: Long-chain carbs Polysaccharides- polymers: poly= “many” meros (Gk) = part Examples: Plant starches cornstarch Glycogen Monomers attach to each other to form polymers! Starch Glucose Figure 2-13A Starch Lipids Contain carbon and hydrogen atoms Usually fats, oils, and waxes Used to store energy or build membranes Nucleic acids Contain hydrogen, oxygen, nitrogen, carbon and phosphorus Transmit heredity (genetic information) DNA (deoxyribonucleic acid) and RNA Monomer: nucleotide Proteins Contain N, O, C, H, Made of monomers (smaller molecules) called amino acids Control rate of reactions, regulate cell processes, form tissues, transport substances, fight disease. Section 2-3 Protein Structure Amino acids Figure 2-17 A Protein One type of protein… enzymes http://highered.mcgrawhill.com/sites/0072495855/student_view0/chapter2/a nimation__how_enzymes_work.html Homework Set up Enzyme Lab in your lab book. Be ready to go tomorrow! Chemical Testing We can test for the presence of these compounds in food by using CHEMICAL REAGENTS. These chemical reagents are chemicals that react in a particular way in the presence of these nutrients. Carbs: sugars, starches Proteins Lipids In your notes… Nutrient Observations Reagent/Test Simple Sugar (monosaccharide) Carbohydrates Starch (polysaccharide) Lipids Proteins Positive Negative Testing for Starch (polysaccharide) Which sample is negative for starch? Which one is positive for starch? Testing for Sugar (monosaccharide): Which sample is positive for starch? Which sample is positive for sugar? Which sample is positive for a monosaccharide? Testing for Proteins: Which sample shows a presence of amino acid chains? Which sample shows a presence of a polymer? Testing for Lipids: Brown bag test