* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Metabolizma - mustafaaltinisik.org.uk
Survey
Document related concepts
Transcript
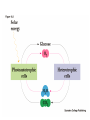
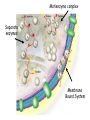
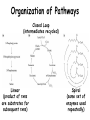
Introduction to Metabolism Metabolism • The sum of the chemical changes that convert nutrients into energy and the chemically complex products of cells • Hundreds of enzyme reactions organized into discrete pathways • Substrates are transformed to products via many specific intermediates • Metabolic maps portray the reactions • Intermediary metabolism A Common Set of Pathways • Organisms show a marked similarity in their major metabolic pathways • Evidence that all life descended from a common ancestral form • There is also significant diversity The Sun is Energy for Life • Phototrophs use light to drive synthesis of organic molecules • Heterotrophs use these as building blocks • CO2, O2, and H2O are recycled Metabolism • Metabolism consists of catabolism and anabolism • Catabolism: degradative pathways – Usually energy-yielding! • Anabolism: biosynthetic pathways – energy-requiring! Catabolism and Anabolism • Catabolic pathways converge to a few end products • Anabolic pathways diverge to synthesize many biomolecules • Some pathways serve both in catabolism and anabolism • Such pathways are amphibolic Organization in Pathways • • • • Pathways consist of sequential steps The enzymes may be separate Or may form a multienzyme complex Or may be a membrane-bound system • New research indicates that multienzyme complexes are more common than once thought Mutienzyme complex Separate enzymes Membrane Bound System Organization of Pathways Closed Loop (intermediates recycled) Linear (product of rxns are substrates for subsequent rxns) Spiral (same set of enzymes used repeatedly) Metabolism Proceeds in Discrete Steps •Enzyme specificity defines biosynthetic route •Controls energy input and output •Allow for the establishment of control points. •Allows for interaction between pathways Regulation of Metabolic Pathways • Pathways are regulated to allow the organism to respond to changing conditions. • Most regulatory response occur in millisecond time frames. • Most metabolic pathways are irreversible under physiological conditions. • Regulation ensures unidirectional nature of pathways. • Flow of material thru a pathway is referred to as flux. • Flux is regulated by supply of substrates, removal of products, and activity of enzymes Enzyme Regulation of Flux Common mechanisms • feedback inhibition – product of pathway down regulates activity of early step in pathway • Feedforward activation – metabolite produced early in pathway activates down stream enzyme Metabolic Control Theory • Pathway flux is regulated by multiple enzymes in a pathway. • Control coefficient determined for each enzyme. = D activity / D enzyme concentration. • Enzymes with large control coefficients impt to overall regulation. • Recent finding suggest that the control of most pathways is shared by multiple pathwayt enzymes Regulating Related Catabolic and Anabolic Pathways • Anabolic & catabolic pathways involving the same compounds are not the same • Some steps may be common to both • Others must be different - to ensure that each pathway is spontaneous • This also allows regulation mechanisms to turn one pathway and the other off Metabolic Pathways are not at Equilibrium • Metabolic pathways are not at equilibrium A <-> B • Instead pathways are at steady state. A -> B -> C The rate of formation of B = rate of utilization of B. Maintains concentration of B at constant level. All pathway intermediates are in steady state. Concentration of intermediates remains constant even as flux changes. Thermodynamics and Metabolism • Standard free energy A + B <-> C + D • DGo’ =-RT ln[C][D]/[A][B] • DGo’ = -RT ln Keq • DGo’ < 0 (Keq>1.0) Spontaneous forward rxn • DGo’ = 0 (Keq=1.0) Equilibrium • DGo’ > 0 (Keq <1.0) Rxn requires input of energy DG (not DGo’) is impt in vivo • DG = DGo’ + RT ln Q • Q (mass action ratio) = [C]’[D]’/[A]’[B]’ • Actual [reactants] and [products] used to determine Q. • Because reactions are at steady state not equilibrium, Q does not equal Keq • When Q is close in value to Keq = nearequilibrium rxn (reversible) • If Q is far from Keq = metabolically irreversible rxn. • ATP is the energy currency of cells • In phototrophs, light energy is transformed into the light energy of ATP • In heterotrophs, catabolism produces ATP, which drives activities of cells • ATP cycle carries energy from photosynthesis or catabolism to the energyrequiring processes of cells ATP Phosphoric Acid Anhydrides • ADP and ATP are examples of phosphoric acid anhydrides • Large negative free energy change on hydrolysis is due to: – electrostatic repulsion – stabilization of products by ionization and resonance – entropy factors Phosphoryl-group Transfer • Energy produced from a rxn can be coupled to another rxn that requires energy to proceed. • Transfer of a phosphate group from high energy phosphorylated compounds can activate a substrate or intermediate of an energy requiring rxn. A-P + ADP -> A + ATP, ATP +C-> ADP + C-P • The ability of a phosphorylated compound to transfer a phosphoryl group is termed its phosphoryl-group-transfer-potential. Phosphoryl-group Transfer