* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Diapositive 1
Survey
Document related concepts
Transcript
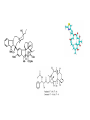
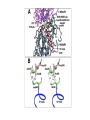
Dimère alpha-beta tubuline Paclitaxel and docetaxel • Paclitaxel is currently regarded as one of the best new anticancer agents (1-5). The drug was approved by the U.S. Food and Drug Administration (FDA) for the treatment of advanced ovarian cancer in 1992 and breast cancer in 1994. Since the isolation of paclitaxel from the bark of the Pacific yew, Taxus brevifolia, in 1966, enormous research has been performed on the drug's applications. The attention of the medical world turned toward this new natural product in 1979 when Susan B. Horwitz and co-workers reported paclitaxel's unique mechanism in stopping the proliferation of cancer cells (6, 7). • Paclitaxel is the first anticancer agent that promotes tubulin assembly (a dynamic process involved in cell proliferation) and stabilizes the resulting microtubules. Because the cell cannot break down the microtubules, it is incapable of going through the whole cell cycle, and so the cell ultimately dies. Paclitaxel is currently undergoing clinical trials for treatment of lung, head, neck, and gastrointestinal cancers. A semisynthetic analogue called docetaxel (generic name for Taxotere), developed by Rhône-Poulenc Rorer, was approved by the FDA in 1996 for the treatment of breast cancer (8). The epothilones are a novel class of non-taxane microtubule-stabilizing agents obtained from the fermentation of the cellulose degrading myxobacteria, Sorangium cellulosum. Preclinical studies have shown that the epothilones are more potent than the taxanes and active in some taxane-resistant models. Similar to paclitaxel and other taxanes, the epothilones block cells in mitosis, resulting in cell death. The chief components of the fermentation process are epothilones A and B, with epothilones C and D found in smaller amounts. Trace amounts of other epothilones have also been detected. Pre-clinical studies have shown that epothilone B is the most active form, exhibiting significantly higher antitumor activity than paclitaxel and docetaxel. Several phase I and phase II clinical trials are ongoing with epothilone B and BMS 247550, an epothilone B analog. Preliminary reports indicate these agents are active against human cancers in heavily pre-treated patients. The epothilones appear to be well tolerated, with a side effect profile that is similar to that reported with the taxanes. TOXICITY: The two commercially available taxanes (paclitaxel and docetaxel) are widely employed in standard oncologic practice. Toxicity of the agents includes bone marrow suppression (principally neutropenia), complete alopecia, and hypersensitivity reactions. While both drugs can cause neurotoxicity and myalgias/arthralgias, this is a greater clinical concern with paclitaxel. Docetaxel can be associated with the development of significant fluid retention (e.g., edema, ascites, pleural effusions), the incidence and severity of which appear to be limited by prophylactic treatment with corticosteroids both before and after each treatment. If patients are monitored closely (e.g., for hypersensitivity reactions, bone marrow suppression) the taxanes have a favorable side effect profile, and it is currently uncommon for treatment to be discontinued because of the development of excessive toxicity.