* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Paclitaxel
Compounding wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
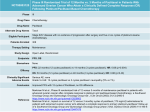
P Paclitaxel NH CH3 O OH O O O O 10 9 11 12 876 5 4 3 O OO O O OH CH H CH3 O 12 13 14 r rr Trade Names Taxol Classification Taxane, anti-microtubule agent Category Chemotherapy drug Drug Manufacturer Bristol-Myers Squibb Mechanism of Action Isolated from the bark of the Pacific yew tree, Taxus brevifolia . Cell cycle–specific, active in the mitosis (M) phase of the cell cycle. High-affinity binding to microtubules enhances tubulin polymerization. Normal dynamic process of microtubule network is inhibited, leading to inhibition of mitosis and cell division. Mechanism of Resistance Alterations in tubulin with decreased binding affinity for drug. Multidrug-resistant phenotype with increased expression of P170 glycoprotein. Results in enhanced drug efflux with decreased intracellular accumulation of drug. Cross-resistant to other natural products, including vinca alkaloids, anthracyclines, taxanes, and VP-16. Absorption Poorly soluble and not orally bioavailable. Distribution Distributes widely to all body tissues, including third-space fluid collections such as ascites. Negligible penetration into the CNS. Extensive binding (_90%) to plasma and cellular proteins. Metabolism Metabolized extensively by the hepatic P450 microsomal system. About 70%–80% of drug is excreted via fecal elimination. Less than 10% is eliminated as the parent form with the majority being eliminated metabolites. Renal clearance is relatively minor with less than 10% of drug cleared via the kidneys. Terminal elimination half-life ranges from 9 to 50 hours depending on the schedule of administration. Indications Ovarian cancer. Breast cancer. Non–small cell and small cell lung cancer. Head and neck cancer. Esophageal cancer. Prostate cancer. Bladder cancer. AIDS-related Kaposi’s sarcoma. Dosage Range Ovarian cancer: 135–175 mg/m 2 IV as a 3-hour infusion every 3 weeks. Breast cancer: 175 mg/m 2 IV as a 3-hour infusion every 3 weeks. Bladder cancer, head and neck cancer: 250 mg/m 2 IV as a 24-hour infusion every 3 weeks. Weekly schedule: 80–100 mg/m 2 IV each week for 3 weeks with 1 week rest. Infusional schedule: 140 mg/m 2 as a 96-hour infusion. Drug Interaction 1 Radiation therapy—Paclitaxel is a radiosensitizing agent. Drug Interaction 2 Concomitant use of inhibitors and/or activators of the liver cytochrome P450 CYP3A4 enzyme system may affect paclitaxel metabolism and its subsequent antitumor and host toxic effects. Drug Interaction 3 Phenytoin, phenobarbital—Accelerate the metabolism of paclitaxel resulting in lower plasma levels of drug. Drug Interaction 4 Cisplatin, carboplatin—Myelosuppression is greater when platinum compound is administered before paclitaxel. Platinum compounds inhibit plasma clearance of paclitaxel. When a platinum analog is used in combination, paclitaxel must be given first. Drug Interaction 5 Cyclophosphamide—Myelosuppression is greater when cyclophosphamide is administered before paclitaxel. Drug Interaction 6 Doxorubicin—Paclitaxel reduces the plasma clearance of doxorubicin by 30%–35%, resulting in increased severity of myelosuppression. Special Considerations Contraindicated in patients with history of severe hypersensitivity reaction to paclitaxel or to other drugs formulated in Cremophor EL, including cyclosporine, etoposide, or teniposide. Use with caution in patients with abnormal liver function. Dose reduction is required in this setting. Patients with abnormal liver function are at significantly higher risk for host toxicity. Contraindicated in patients with severe hepatic dysfunction. Use with caution in patients with prior history of diabetes mellitus and chronic alcoholism or prior therapy with known neurotoxic agents such as cisplatin. Use with caution in patients with previous history of ischemic heart disease, with myocardial infarction within the preceding 6 months, conduction system abnormalities, or on medications known to alter cardiac conduction (beta blockers, calcium channel blockers, and digoxin). Patients should receive premedication to prevent the incidence of hypersensitivity reactions (HSR). Give dexamethasone 20 mg PO at 12 and 6 hours before drug administration, diphenhydramine 50 mg IV, and cimetidine 300 mg IV at 30 minutes before drug administration. Patients experiencing major HSR may be rechallenged after receiving multiple high doses of steroids, dexamethasone 20 mg IV every 6 hr for 4 doses. Patients should also be treated with diphenhydramine 50 mg IV and cimetidine 300 mg IV 30 minutes before the rechallenge. Medical personnel should be readily available at the time of drug administration. Emergency equipment, including Ambu bag, EKG machine, IV fluids, pressors, and other drugs for resuscitation, must be at bedside before initiation of treatment. Monitor patient’s vital signs every 15 minutes during the first hour of drug administration. HSR usually occurs within 2–3 minutes of start of infusion and almost always within the first 10 minutes. Patients who have received _6 courses of weekly paclitaxel should be advised to avoid exposure of their skin as well as their fingernails and toenails to the sun as they are at increased risk for developing onycholysis. This side effect is not observed with the every-3-week schedule. Pregnancy category D. Breast-feeding should be avoided. Toxicity 1 Myelosuppression. Dose-limiting neutropenia with nadir at day 8–10 and recovery by day 15–21. Decreased incidence of neutropenia with 3-hour schedule when compared to 24-hour schedule. Toxicity 2 HSR. Occurs in up to 20%–40% of patients. Characterized by generalized skin rash, flushing, erythema, hypotension, dyspnea, and/or bronchospasm. Usually occurs within the first 2–3 minutes of an infusion and almost always within the first 10 minutes. Incidence of HSR is the same with 3- and 24-hour schedules. Premedication regimen, as outlined in Special Considerations, has significantly decreased incidence. Toxicity 3 Neurotoxicity mainly in the form of sensory neuropathy with numbness and paresthesias. Dose-dependent effect. Other risk factors include prior exposure to known neurotoxic agents (e.g., cisplatin) and pre-existing medical disorders such as diabetes mellitus and chronic alcoholism. Also more frequent with longer infusions and at doses _175 mg/m 2 . Motor and autonomic neuropathy observed at high doses. Optic nerve disturbances with scintillating scotomata observed rarely. Toxicity 4 Transient asymptomatic sinus bradycardia is most commonly observed cardiotoxicity. Occurs in 30% of patients. Other rhythm disturbances are seen, including Mobitz type I, Mobitz type II, and third-degree heart block, as well as ventricular arrhythmias. Toxicity 5 Alopecia. Occurs in nearly all patients, with loss of total body hair. Toxicity 6 Mucositis and/or diarrhea seen in 30%–40% of patients. Mucositis is more common with the 24-hour schedule. Mild to moderate nausea and vomiting, usually of brief duration. Toxicity 7 Transient elevations in serum transaminases, bilirubin, and alkaline phosphatase. Toxicity 8 Onycholysis. Mainly observed in those receiving _6 courses on the weekly schedule. Not seen with the every-3-week schedule.