* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Bridging Studies
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Clinical trial wikipedia , lookup
Pharmacokinetics wikipedia , lookup
1
Chapter 9
Bridging Studies
2
Outline
Introduction
Taiwan’s Situations
An Bayesian Approach
Discussion
3
Introduction
ICH (International Conference on
Harmonisation) E5
Ethnic Factors in the Acceptability of
Foreign Clinical Data
The purpose of this guidance is to facilitate the
registration of medicines among ICH regions
by recommending a framework for evaluating
the impact of ethnic factors upon a medicine’s
effect, i.e., its efficacy and safety at a particular
dosage and dose regimen.
4
Ethnic Difference
Patients surviving (%)
—— IRESSA®
------ Placebo
1.0
1.0
1.0
0.9
0.9
Asian (n = 342)
0.9
HR = 0.66 (0.48, 0.91), P = .011
0.8
0.8
0.8
RR = 12.0%
0.7
0.7
0.6
0.5
0.5
0.5
0.4
0.4
0.4
0.3
0.3
0.3
0.2
0.2
0.2
0.1
0.1
0.1
0 0 11 22 33 44 55
RR = 6.5%
0.7
0.6
0.6
0.0
0.0
Non-Asian (n = 1350)
HR = 0.93 (0.81, 1.08), P = .364
0.0
6
7
8
9
10
11
12
13
14
6 7 8 9 10 11 12 13 14 15 1500 11
22 33
Time, mo
44 55
6 77 88 99 10
10 11
1112
12 13
1314
14 15
15
6
5
Objectives of ICH E5
To describe the characteristics of foreign clinical data that
will facilitate their extrapolation to different populations
and support their acceptance as a basis for registration of a
medicine in a new region
To describe regulatory strategies that minimize duplication
of clinical data and facilitate acceptance of foreign clinical
data in the new region
To describe the use of bridging studies, when necessary, to
allow extrapolation of foreign clinical data to a new region
To describe development strategies capable of
characterizing ethnic factor influences on safety, efficacy,
dosage, and dose regimen
6
Bridging Data Package
A bridging data package consists of
1) Selected information from the complete clinical
data package (CCDP) that is relevant to the
population of the new region, including
pharmacokinetic data, and any preliminary
pharmacodynamic and dose-response data,
and
2) If needed, a bridging study to extrapolate the
foreign efficacy and/or safety data to the new
region.
7
Complete Clinical Data
Package
A clinical data package intended for
registration containing clinical data that fulfill the
regulatory requirements of the new region and
containing pharmacokinetic data relevant to the
population in the new region
8
Bridging Study
A bridging study is defined as a supplemental study
performed in the new region to provide
pharmacodynamic or clinical data on efficacy,
safety, dosage, and dose regimen in the new
region that will allow extrapolation of the
foreign clinical data to the new region.
9
Ethnic Factors
Intrinsic Ethnic Factors are more genetic and
physiologic in nature
e.g., genetic polymorphism, age, gender, height,
weight, lean body mass, body composition, and
disease conditions, etc.
Extrinsic Ethnic Factors are more social and
cultural in nature
e.g., environment, culture, medical practice, health
insurance, practices in clinical trials or conduct
10
Bridging Studies
• ICH E5
• Only after the medicine is approved in
the original region
• Performed in the new region
11
Taiwan’s Situations
12
Taiwan Before Bridging Study
An approved local clinical trial study report is
required for the new drug application in
Taiwan—July 7 Announcement in 1993
Disadvantage:
A sample size of 40 as required would be
difficult to demonstrate significant importance
clinically or statistically
The study design of the local trial usually only
repeated a study that has been done in the foreign
countries but in a smaller sample size;The study
has not been designed based on the medical
situation in Taiwan
13
Taiwan’s Strategy to Implement
Bridging Study
Smoothly convert compulsory Local Clinical Trial (LCT) to
meaningful bridging study
Gradually, stepwise announce waived local clinical trial
Create an environment: (1) meet international regulation,
ICH
(2) require optimized dosage for
Taiwanese patient
Communicate with local and international pharmaceutical
industry
Announce new regulation according to the international
norm and the consensus from communications
Create an international platform “APEC – Taipei”
Implement Double Twelve Announcement – Bridging
Study
14
Stepwise Implementation
1998 Announce: two years later, switch from LCT to
bridging study
Many communications and negotiations with local and
international pharmaceutical industry
2000, Dec.12, (Double Twelve Announcement) – public
announce bridging study regulation
1998 Five announcements of LCT wavier
Two years transition periods: both LCT and bridging
studies acceptable from 2000 ~ 2002
Many international conferences held in Taipei and other
Asian countries, regarding BS, through the APEC platform
Ask CDE to complete the practical issues related to
implementation of BS
2004, Jan. 1, Bridging evaluation
15
Available Statistical Methods
1. Hierarchical Model
(Liu, Hsueh, and Chen, 2002, Biometrical Journal,
44: 969-981)
2. Takeuchi, M. Controlled Clinical Trials 23: 55-57, 2002
3. Shao, J. and Chow, S. C. Statistics in Medicine, 21:
1727-1742, 2002
4. Population Similarity
(Chow, Shao, Hu, 2002, JBS, 12: 385-400)
5. Consistency Approach
(Shih, 2001, Controlled Clinical Trials, 22: 357-366)
6. Bayesian Positive Treatment Approach
(Liu, Hsiao, and Hsueh, 2002, JBS 12: 297-294)
7. Bayesian Noninferiority Approach
(Liu, Hsueh and Hsiao, 2004, JBS accepted)
8. Group Sequential Approach
(Hsiao, Xu and Liu, 2003, JBS, 13: 793-801)
9. Two-Stage Approach
(Hsiao, Xu and Liu, 2004, submitted)
Checking List for Sponsors (1 of 3)
Checking List for the evaluation of Bridging Study by the Sponsor
INFO
Data Package
Y3 N
Vol., page1
I. The current status of clinical study of the drug in the world
□□
II. NDA expert report or Investigator’s Brochure2
□□
III. Pharmacokinetics, safety and efficacy data related to Asian population
□□
IV.Comparative analysis of Pharmacokinetics, safety and efficacy data
between Asian population and others.
□□
V. Self evaluation (please provide reference materials or literature)
Y N U
□□
□□□
□□
2. Is the drug with a steep pharmacodynamic curve for both efficacy
and safety (a small change in dose results in a large change in
□□□
effect) in the range of the recommended dosage and dose
regimen?
□□
□□□
□□
1. Does the drug show a Non-linear pharmacokinetics at the
therapeutic dose?
3. Is the drug with narrow therapeutic dose range?
Note:
1. To speed up reviewing process, please clearly indicate the volume and page number as requested. In
addition to the page number, the related paragraph may be highlighted when necessary.
2. Please provide the comparative analysis of different ethnic groups, if it’s available. Please also explain
if there is no comparative analysis of different ethnic groups in NDA expert report.
3. Y=yes; N=no; U=unknown
16
17
Checking List For Sponsors (2 of 3)
Checking List for the evaluation of Bridging Study by the Sponsor
V. Self evaluation (please provide reference materials or literature)
4. Is the drug highly metabolized, especially through a single
pathway, thereby increasing the potential for drug-drug
interaction ?
5. Is the drug metabolized by enzyme known to show genetic
polymorphism?
INFO
Data Package
Y2 N
Vol., page1
Y N U
□□
□□□
□□
□□□
□□
6.
Is the drug administered as a prodrug, with the potential for
ethnically variable enzymatic conversion ?
□□□
□□
7.
Is the drug with high inter-subject variation in bioavailability ?
□□□
□□
8.
Is the drug with low bioavailability, thus more susceptible to
dietary absorption effects?
□□□
□□
9.
Is the drug with high likelihood of use in setting of multiple comedications ?
□□□
□□
□□□
□□
10. Is the drug with high likelihood for inappropriate use, e.g.
analgesics and tranquilizers ?
Note:
1.
To speed up reviewing process, please clearly indicate the volume and page number as requested. In
addition to the page number, the related paragraph may be highlighted when necessary.
2.
Y=yes; N=no; U=unknown
Checking List for Sponsors (3 of 3)
Checking List for the evaluation of Bridging Study by the Sponsor
V. Self evaluation (please provide reference materials or literature)
INFO
Data Package
Y3 N
Vol., page1
Y N U
□□
11. Is there any difference in epidemics of applied indication between
the major study population and our population (including medical
□□□
history, mechanism of disease development and the rate of
occurrence, the efficacy and safety of other drugs in the same
class)?
□□
12. Other important ethnic sensitive factors, such as “Is there any
difference in the medical practice?”
□□
□□□
VI. Post-marketing surveillance information
□□
Overall conclusion of self evaluation (Is it clinically insignificant? What
is the risk and benefit of the drug applied (such as, “Does the indication
applied belong to severe disease”, “Is there a alternative therapy?”, “Are
the differences of the data in ethnic factors acceptable ?)
□□
Summary3
□□
Note:
1. To speed up reviewing process, please clearly indicate the volume and page number as requested. In
addition to the page number, the related paragraph may be highlighted when necessary.
2. Y=yes; N=no; U=unknown
3. Please according the checking list provide an integrate summary or a brief description of all the
information submitted.
18
19
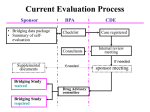
Sponsor
•Bridging Data Package
•Summary for the
Consideration of Bridging
Study
BoPA
Accept
submission
Checking List
Expert Consultants
(Statistical, Clinical,
Pharmacokinetics
reviewers)
CDE
CDE acceptance
verification
Technical Review
(Designate
reviewer)
Review meeting
Schedule Sponsor
meeting
Supplement
Result of Evaluation:
1. No Bridging study
required
2. Bridging study is required
– Type of Bridging study
Sponsor meeting
Clinical Review
Committee
Notification
Review report and
Recommendation:
1. No Bridging
study required
2. Bridging study is
required – Type of
Bridging study
20
m
ph
ar
ne
eq
u
at
e
/in
rie
n
su
f fi
xp
e
cie
n
lo
gy
rn
at
a
40
td
As
ia
io
in
em
ce
ep
id
ct
er
n
nc
ef
fe
co
nc
e
co
d
21.1
ef
fic
ac
y
sa
fe
ty
fo
o
ic
s
tio
n
ac
te
r
21.1
in
ad
in
et
ic
s
od
yn
am
ac
m
ug
ok
in
Reasons for not waived/total cases (%)
60
re
s/
ph
ar
tic
dr
ug
-
dr
ac
m
80
un
fa
m
ilia
ok
i
ac
ph
ar
21
100
71.1
55.3
47.4
31.6
21.1
20
5.3
2.6
0
22
Does bridging strategy of ICH E5 warrant
further implementation?
Is Taiwan on the right way?
23
Case I
Drug A is a fixed combination of two anti-platelet
agents with indication for secondary prevention of
thromboembolic stroke (200mg
dipyridamole/25mg aspirin 1bid)
After the standard process of BSE, we decided to
request a bridging study due to an ethnic
difference in medical practice (much lower dose
for one of the components in Taiwan) and higher
headache-associated dropout rate in previous
Philippine study
24
Case I
Headache drop out rate: Phillipino > Caucasian
Local Bridging Study Result : first 4 weeks
Group
Placebo
Reduced Dose 2wk
Full Dose
Full Dose 2wk
4wk
Headache
8.7%
6.7%
16.3%
drop out rate
Risk Management: Change labeling’s instruction for use
25
Case II
Drug B is a new potent lipid-lowering agent
The PK study in Japanese shows that Cmax
of Japanese is 1.9~2.5 times of that for
Caucasian while AUC is 2~2.5 times
Although the mean interracial difference is
not substantial, Taiwan approved the drug
with reduced maximal dosage due to the
dose-dependent, drug-related rare SAE of
rhabdomyolysis
26
Case II
The decision is further echoed by US FDA
After reviewing the results of a Phase IV
PK study in Asian-Americans, FDA urged
the physician to reduce the starting dose and
prescribe high dose with caution for Asians
in Labeling in March, 2005
27
Bayesian Approach
28
Bayesian Approach
For bridging studies
Small sample size
No power
Information on dose response, efficacy and safety of the
original region can not be concurrently obtained from the
local bridging studies but are available in the trials
conducted in the original region
Need to borrow “strength” from CCDP of the original
region
Information on dose response, efficacy and safety of the
original region can and should be incorporated in a
statistically sound manner to evaluate bridging evidence by
local bridging studies
29
Bayesian Approach
Before Experiment
Past
experience about
similar situations Prior information
P(q )
involving
similar q
Treatment
effect
q
After Experiment
Observed results
( Data )
Posterior
information
P(q| prior&data)
Make
Statistical
inference
30
Assumption, Notation and
Hypotheses
We focus on the trials for comparing a test product
and a placebo control
Xi and Yj are some efficacy responses for patients i
and j receiving the test product and the placebo
control respectively in the new region
Xi’s and Yj’s are normally distributed with known
variance σ2
μNT and μNP are the population means of the test
and placebo, respectively, and let ΔN = μNT - μNP
H0: ΔN 0 vs. HA: ΔN > 0
31
Parameters
Test Product
Effect
Placebo Effect
Original Region
m OT
m OP
New Region
m NT
m NP
32
Before Bridging Study
Original region data
to estimate m OT
Original region data
to estimate m OP
Conclude
m OT > m OP
33
Bayesian Positive Treatment
Approach
Original region
information to
form prior
New region
treatment effect
m NT — m NP
Bridging data
Posterior
information
P{mNT-mNP |prior & bridging}
Conclude mNT>mNP if
P{mNT-mNP>0 |prior & bridging}
is large
34
Previous Statistical Approach
Use the estimate of treatment effect from
the original region formulated as a normal
prior
Compute the posterior treatment effect with
the data from the new region
35
Previous Statistical Approach
Results from Original Region
Change from baseline in sitting DBP at week 12
Region Statistics
Test
Placebo
I
II
III
n
Mean
SD
n
Mean
SD
n
Mean
SD
138
-18
11
185
-17
10
141
-15
13
132
-3
12
179
-2
11
143
-5
14
36
Previous Statistical Approach
Results from New Region:
Change from baseline in Sitting DBP at week 12
Region Statistics
Test
Placebo
New
64
-4.5
11
65
-3.8
11
n
Mean
SD
Posterior probability of similarity: Psp 1
37
Previous Statistical Approach
Original region: Efficacy of the test drug is
superior to the placebo
New Region: Reduction of sitting BP of the test
drug is same as that of the placebo
Conclusion: The results of the original region can
be extrapolated to the new region despite of
inconsistent results between original and new
regions
Evaluation of bridging studies is overwhelmingly
by the results of original region due to imbalance
of information provided by the two regions
38
Use of Prior Distribution
The proposed mixture model of the prior
distribution for ΔN is a weighted average of
the noninformative and normal priors as given
below
π(ΔN) =γπ1(ΔN) + (1-γ)π2(ΔN)
π1(.) ≡c is a non-informative prior
π2(.) is a normal prior with mean θ0 and variance
σ02 which summarizes the foreign clinical data
about the treatment difference provided in the
CCDP
0≦γ≦1
39
Marginal Density
Based on the clinical responses from the
bridging study in new region, ΔN can be
estimated by
ˆ x y .
N
N
N
The marginal density is
(ˆ N q 0 ) 2
m(ˆ N ) (1 )
exp
,
2
2
~
2( 0 )
2 ( 02 ~ 2 )
1
where
~ 2 2 / nT 2 / nP .
40
Posterior Distribution
Given the bridging data and prior distribution,
the posterior distribution of ΔN is
1
ˆ
( N | N )
m(ˆ
)
( N ˆ N ) 2
exp
2
~
~
2
2
1
2
2
ˆ
(
q
)
(
)
1
N
0
N
N
(1 )
exp
.
2
2
~
~
2 0
2
2 0
41
Bridging Evaluation
Similarity on efficacy in terms of a positive
treatment effect for the new region can be
concluded if the posterior probability of
Similarity
PSP P( m NT m NP 0 | bridging data and prior )
( N | ˆ N )d N
0
1,
for some pre-specified 0 < < 0.5.
42
Example
The CCDP provides the results of three
randomized, placebo controlled trials for a
new antidepressant (test drug) conducted in
the original region
The primary endpoint is the change from
baseline of sitting diastolic blood pressure
(mmHg) at week 12
A bridging study was conducted in the new
region to compare the difference in efficacy
between the new and original region
43
Three Scenarios
The first scenario presents the situation where no
statistically significant difference in the primary
endpoint exists between the test drug and placebo (2sided p-value = 0.6430
The second situation is that the mean reduction of
sitting diastolic blood pressure at week 12 of the test
drug is statistically significantly greater than the
placebo group (2-sided p-value < 0.0001)
The third scenario is the situation where due to the
insufficient sample size of the bridging study, no
statistical significance is found between the test drug
and placebo although the magnitude of the difference
between the test drug and placebo observed in the
original region is preserved in the new region (2-sided
p-value = 0.0716)
Region
Statistics
44
Treatment Group
Drug
Placebo
N
138
132
Mean
-18
-3
Standard Deviation
11
12
N
185
179
Mean
-17
-2
Standard Deviation
10
11
N
141
143
Mean
-15
-5
Standard Deviation
13
14
New 1
N
64
65
(Example 1)
Mean
-4.7
-3.8
Standard Deviation
11
11
New 2
N
64
65
(Example 2)
Mean
-15
-2
Standard Deviation
11
11
New 3
N
24
23
(Example 3)
Mean
-11
-4
Standard Deviation
13
13
Original 1
Original 2
Original 3
45
0.0
0.1
0.2
0.3
0.4
0.5
0.6
0.7
0.8
0.9
1.0
Example 1
1.0000
0.6789
0.6789
0.6789
0.6789
0.6789
0.6789
0.6789
0.6789
0.6789
0.6789
Psp
Example 2
1.0000
0.9999
0.9999
0.9999
0.9999
0.9999
0.9999
0.9999
0.9999
0.9999
0.9999
Example 3
1.0000
0.9727
0.9700
0.9690
0.9685
0.9682
0.9680
0.9678
0.9677
0.9676
0.9675
46
Scenario I
If the regulatory agency allows all
information of the original region to be used
for evaluation of similarity between the new
and original region, γ is set to be 0 and
hence PSP 1.00
If γ ≧ 0.1, then PSP always drops to around
0.6789
47
Scenario II
The values of PSP in Example 2 appear to be
close to 1.00 regardless of the choice of γ
48
Scenario III
• The values of PSP are all greater than 0.9675 for all
values of γ between 0 and 1
• With the strength of the substantial evidence of
efficacy is borrowed from the CCDP of the
original region, our procedure can prove the
similarity of efficacy between the new and original
region when a non-significant efficacy result but
with a similar magnitude is observed in the
bridging study
49
Final Remarks
The proposed prior is a weighted average of a
non-informative prior and a normal prior
The proposed procedure can avoid the situation of
concluding similarity between the new and
original region when the efficacy result of the test
drug observed the bridging study of the new
region is same as or even worse than that of the
placebo group
Our proposed procedure can reach a conclusion
that is more consistent with the results obtained
from the bridging study
50
Final Remarks
Selection of weight γ by the regulatory
agency in the new region should consider
all differences in both intrinsic and extrinsic
ethnical factors between the new and
original regions and at the same time should
also reflect their belief on the evidence of
efficacy provided in the CCDP of the
original region
51
Final Remarks
We use a normal prior for summarization of
the results in CCDP of the original region
We also use other prior distributions
I) double exponential distribution
II) lognormal distribution
Other different distributions used for π2
reach the same conclusion