* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Checklist
Survey
Document related concepts
Transcript
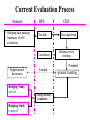
Current Evaluation Process Sponsor • Bridging data package • Summary of selfevaluation BPA Checklist Consultants CDE Case registered Internal review meeting If needed Supplemental documents Bridging Study waived Bridging Study required If needed Drug Advisory committee sponsor meeting “Working Lists” PK/PD Properties and Clinical Properties for Assessing Ethnic Sensitivity -- PK/PD 1. Is there a non-linear pharmacokinetics? 2. Is there a steep pharmacodynamic curve for both efficacy and safety in the range of the recommended dosage and dose regimen ? 3. Is the therapeutic dose range narrow ? 4. Is it highly metabolized, especially through a single pathway ? 5. Is it metabolized by enzymes known to be genetically polymorphic ? 6. Is it administered as a prodrug ? 7. Dose it show high inter-subject variation in bioavailability ? 8. Is it low in bioavailability ? “Working Lists” PK/PD Properties and Clinical Properties for Assessing Ethnic Sensitivity -- Clinical 9. Is it likely to be used in a setting of multiple comedications ? 10. Is it prone to be used inappropriately? 11. Is there any epidemiologic difference concerning the indication between the reference population and ours ? 12. Other important ethnic sensitive factors ? Checklist Checklist for the Evaluation of Bridging Study by the Sponsor Info provided Reference Y Vo1./Page N I. The current worldwide regulatory status of the drug • • II. NDA expert report or Investigator’s Brochure • • III. Pharmacokinetics, safety and efficacy data of Asian Population • • IV. Comparison of the above data with those of other population • • V. Self evaluation • • VI. Post-marketing surveillance data • • • • VII. Overall conclusion Checklist V.Self evaluation Y N U Info Provided Y N 1. Is there a non-linear pharmacokinetics? • • • • • 2. Is there a steep pharmacodynamic curve for both efficacy and safety in the range of the recommended dosage and dose regimen ? • • • • • 3. Is the therapeutic dose range narrow ? • • • • • 4. Is it highly metabolized, especially through a single pathway ? • • • • • 5. Is it metabolized by enzymes known to be genetically polymorphic ? • • • • • 6. Is it administered as a prodrug ? • • • • • Reference Vo1./Page Checklist V.Self evaluation Y N U Info Provided Y N 7. Dose it show high inter-subject variation in bioavailability ? • • • • • 8. Is it low in bioavailability ? • • • • • 9. Is it likely to be used in a setting of multiple co-medications ? • • • • • 10. Is it prone to be used inappropriately? • • • • • 11. Is there any epidemiologic difference concerning the indication between the reference population and ours ? • • • • • 12. Other important ethnic sensitive factors ? • • • • • Reference Vo1./Page