* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cell Wall Inhibitor Penicillins
Pharmacognosy wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Prescription costs wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug discovery wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Antibiotics wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
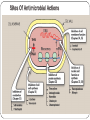
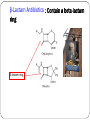
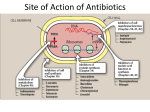
Principle of Antimicrobial & Cell Wall inhibitors General Pharmacology M212 Dr. Laila M. Matalqah Ph.D. Pharmacology Mechanisms of Action of antimicrobials Inhibition of bacterial cell wall synthesis or activation of enzymes that disrupt cell walls (Penicillin, Cephalosporins, Vancomycin) Inhibition of protein synthesis (tetracyclines, clindamycin, aminoglycosides) Inhibition of microbial cell membranes function (antifungals) Inhibition of organism reproduction by interfering nucleic acid synthesis (fluoroquinolones, -antivirals) Inhibition of cell metabolism and growth (sulfonamides) Sites Of Antimicrobial Actions Drug Selection Best if based on culture and sensitivity (the organism’s identity) organism’s susceptibility to a particular agent, MIC: minimum inhibitory concentration—lowest concentration of a drug that prevents visible growth of microorganisms site of the infection: Drug’s ability to penetrate infected tissues (prostate, CNS) Drug’s toxicity and the risk-to-benefit ratio Drug costs patient factors Drug Selection: site of the infection The lipid solubility of a drug: For example, lipid-soluble drugs, such as chloramphenicol and metronidazole, have significant penetration into the CNS. β-lactam antibiotics, such as penicillin, are ionized at physiologic pH and have low solubility in lipids 1. However, In infections such as meningitis the barrier does not function as effectively, and local permeability is increased. Some β-lactam antibiotics can then enter the CSF in therapeutic amounts other factors: Molecular weight of the drug, Protein binding of the drug Drug Selection: patients factors 1. 2. 3. 4. 5. 6. Immune system : Alcoholism, diabetes, AIDS, malnutrition, autoimmune diseases, pregnancy Renal dysfunction: (for example, aminoglycosides? C/I) Hepatic dysfunction: Antibiotics that are concentrated or eliminated by the liver (for example, erythromycin and tetracycline Age : Young children should not be treated with tetracyclines or quinolones, which affect bone growth. Pregnancy Lactation: Antibiotic Combination Therapy Used when infection is caused by multiple microorganisms Serious infections in which a combination is synergistic (aminoglycoside and antipseudomonal penicillin) β-lactam antibiotics are synergistic with the aminoglycosides.?? How? Likely emergence of drug resistant organisms In those who are immunosuppressed Chemotherapeutic Spectra A. Narrow-spectrum antibiotics: acting only on a single or a limited group of microorganisms e.g., Isoniazid is active only against mycobacteria B. Extended-spectrum antibiotics: antibiotics that are effective against gram-positive organisms and also against a significant number of gram-negative bacteria e.g. Ampicillin C. Broad-spectrum antibiotics: affect a wide variety of microbial species and can alter the nature of the normal bacterial flora and precipitate a superinfection of an organism such as Clostridium difficile ex., Tetracycline Antimicrobial characteristics Bacteriostatic: Inhibit the growth and replication of bacteria thus limiting the spread of infection until the body’s immune system attacks, immobilizes, and eliminates the pathogen. Bactericidal: kill bacteria at drug serum levels achievable in the patient Minimum inhibitory concentration (MIC):the lowest concentration of antibiotic that inhibits bacterial growth Minimum bactericidal concentration (MBC) : the minimum concentration of antibiotic that kills the bacteria Determinants of Rational Dosing Concentration-dependent killing: antimicrobial show a significant increase in the rate of bacterial killing as the concentration of antibiotic increases from 4 to 64 fold the MIC of the drug for the infecting organism, e.g., aminoglycosides, Time-dependent killing: The effect is directly proportional to the percentage of TIME the concentration of the antibiotic at the site of infection is ABOVE the MIC. , e.g., β-lactams AND macrolides. Postantibiotic effect: is a persistent suppression of microbial growth that occurs after levels of antibiotic have fallen below the MIC “Time Dependant vs. Conc dependent” Mechanisms of Resistance Enzymatic inactivation: Generating enzymes that inactivate the antibiotic (beta lactamase) : 1) β-lactamases (“penicillinases”) that hydrolytically inactivate the β-lactam ring of penicillins, cephalosporins, and related drugs; 2) acetyltransferases: inactivating chloramphenicol or aminoglycosides; 3) esterases that hydrolyze macrolides Changing structure of target site (beta lactams – change in penicillin-binding protein) Preventing cellular accumulation of antibiotics by altering outer membrane proteins or using efflux pumps Gram negatives possess an outer membrane and cytoplasmic membrane preventing passage of abx through porins Changing the metabolic pathway that is being blocked Cell Wall Inhibitors β-Lactam Antibiotics Penicillins Cephalosporins Carbapenems Monobactams Other Antibiotics Vancomycin Daptomycin β-Lactamase Inhibitors Clavulanic acid, Sulbactam, Tazobactam β-Lactam Antibiotics : Contain a beta-lactam ring β-lactam ring β-Lactam Antibiotics: Penicillins Mechanism of action Inhibit the last step of bacterial cell wall synthesis (transpeptidation or cross-linkage), THEN Cell lysis Most effective when bacterial cells are dividing Note: they are inactive against organisms lack cell wall structure, such as mycobacteria, protozoa, fungi, and viruses. 1. Inactivate penicillin-binding proteins (PBPs) ( bacterial enzymes involved in the synthesis of the cell wall) 2. Inhibition of transpeptidase: Some PBPs catalyze formation of the cross-linkages between peptidoglycan chains Production of autolysins:degradative enzymes Penicillins Range from very narrow spectrum to very broad spectrum depend on their ability to cross the bacterial peptidoglycan cell wall to reach the PBPs Factors : the size, charge, and hydrophobicity of the particular β-lactam antibiotic. Gram-negative micro organisms have an outer lipopolysaccharide membrane (envelope) acts s barrier to the water-soluble penicillins but some have porins to permit transmembrane entry. The β-lactams are BACTERICIDAL The β-lactams are “time-dependent” killers Penicillins Classification 1. Natural penicillins Penicillin G (I.V) , Penicillin V (oral) 2. 3. Extended-spectrum penicillins (Aminopenicillins) Ampicillin, Amoxicillin Antistaphylococcal penicillins Oxacillin, Dicloxacillin, Nafcillin 4. Antipseudomonal penicillins: Ticarcillin, Piperacillin, 1. Natural penicillins obtained from fermentations of the mold Penicillium chrysogenum. Spectrum: gram-positive and gram-negative cocci and grampositive bacilli Penicillins are susceptible to inactivation by β-lactamases (penicillinases). Penicillin G: Available IM, IV Penicillin V: Orally is more acid-stable Long-acting forms Procaine PenG (12 hrs) Benzathine Pen (4 weeks) 2. Extended-spectrum penicillins (Aminopenicillins) Ampicillin and Amoxicillin: I.V and Orally Spectrum: same like Penicillin G but more against gram- negative bacilli. Listeria monocytogenes Enterococcus Dental Prophylaxis . Integral in H. pylori Amoxicillin better tolerated orally 3. Antistaphylococcal penicillins Nafcillin, Oxacillin, And Dicloxacillin are penicillinase-resistant penicillins. Their use is restricted to the treatment of infections caused by penicillinase-producing staphylococci, including methicillin sensitive S. aureus (MSSA) Methicillin withdrawn from market because of interstitial nephritis 4. Antipseudomonal penicillins Carbenicillin Ticarcillin Piperacillin activit against P. Aeruginosa and gram-negative bacilli Formulation of ticarcillin or piperacillin with clavulanic acid or tazobactam, respectively, extends the antimicrobial spectrum of these antibiotics to include penicillinase-producing organisms PHARMACOKINETICS (penicillins) Absorption: decrease by food in the stomach, must be administered 30 min before meals or 2 to 3 hours postprandial Route of administration: Penicillin V, amoxicillin, and amoxicillin combined with clavulanic acid are oral preparations Ticarcillin, piperacillin, and the combinations of ampicillin with sulbactam, ticarcillin with clavulanic acid, and piperacillin with tazobactam, must be administered intravenously (IV) or intramuscularly (IM). Depot forms: Procaine penicillin G and benzathine penicillin G are administered IM and serve as depot forms PHARMACOKINETICS (penicillins) Distribution: Cross Placenta (not teratogenic) Bone, CSF (inflammation) Prostate (insufficient) Clearance : Renal tubular secretion & G.F. Impaired renal function, dose adjustment? Probenecid inhibits the secretion of penicillins by competing for active tubular secretion via the organic acid transporter and, thus, can increase blood levels Adverse reactions: Penicillins Hypersensitivity; penicilloic acid (5%) varies from rash, angioedema to anaphylactic shock. Diarrhea: for extended spectrum, pseudomembranous colitis from clostridium difficiles Nephritis: methicillin \ Withdrawn from market Neurotoxicity: seizures if injected intrathecally Hematologic toxicities; ex. Piperillicin decreased coagulation β-Lactamase Inhibitors Hydrolysis of the β-lactam ring, by β-lactamase destroys the antimicrobial activity of a β-lactam antibiotic β-Lactamase inhibitors, do not have significant antibacterial activity, they inactivate β-lactamases, so, protecting antibiotics Formulation with a β-lactamase inhibitor, such as clavulanic acid or sulbactam, protects amoxicillin or ampicillin, respectively, from enzymatic hydrolysis and extends their antimicrobial spectrum. For example, without the β-lactamase inhibitor, MSSA is resistant to ampicillin and amoxicillin Beta lactamase inhibition combinations: Augmentin® (amoxicillin/clavulanate), Orall Unasyn® (ampicillin/sulbactam) , IV Timentin® (ticaricillin/clavulanate), IV Resistant to penicillin Beta lactamase activity (G+ve and G-ve) Decreased permeability of drugs (efflux mechanism in Kelbsiella pneumonia ) Altered PBP (ex. MRSA) THANK YOU