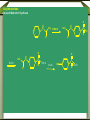* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 2-Sulfonamide & Fluo..
DNA-encoded chemical library wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Ciprofloxacin wikipedia , lookup
Levofloxacin wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
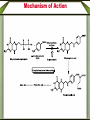
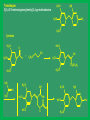
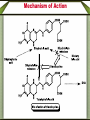
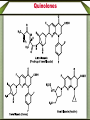
Synthetic antimicrobial agents Synthetic antimicrobial agents have not been modeled after any natural product so they may not properly be called "antibiotics." Some synthetics are extremely effective for treatment of infections and are widely used. They are all effective against key enzymes needed for the biosynthesis of nucleic acids. Because they interrupt the biosynthesis of nucleic acids rather than attacking the finished products or substituting for them in nucleic acids they are not genotoxic but are comparatively safe to use. A- Sulfonamides Sulfonamides were discovered in the mid 1930s following examination of the Prontosoil rubrum dye. It was found that; the active substance is p- aminobenzenesulfonic acid amide (sulfanilamid), formed by reductive liver metabolism of the administered dye i.e. prontosil rubrum is a pro-drug. Sulphonamides General Method of Synthesis O H N NH2 (CH3CO)2O CH3 ClSO3H H N H3C S Cl O O O O H N H3C NH3 S O O O NH2 S H2N O NH2 Sulphonamides General Method of Synthesis O H N CH3 ClSO3H H N H3C S Cl O O O O H N H3C RH2N S O O O S NH-R NaoH H2N O NH-R Mechanism of Action Sulfonamides are bacteriosiatic, they inhibit the enzyme dihydropteroate synthase needed for the biosynthesis of folic acid derivatives and. ultimately, DNA, How? They do this by competing at the active site with paminobenzoic acid (PABA) which incorporated into the developing tetrahydrofolic acid molecule by condensation with a dihydropteroate diphosphate precursor under the influence of dihydropteroate synthetase. Mechanism of Action Mechanism of Action Thus sulfonamides may be classified as antimetabolites Most susceptible bacteria are unable to take up preformed folic acid from their environment and convert it to a tetrahydrofolic acid but, instead, synthesize their own folates de novo. As folates are essential intermediates for the preparation of certain DNA bases, without which bacteria cannot multiply, this inhibition is strongly bacteriostatic. Humans are unable to synthesize folates from component parts, lacking the necessary enzymes (including dihydropteroale synthase), and folic acid is consumed as a dietary so sulfonamides have no lethal effect upon human cell growth. Mechanism of Action In a few strains of bacteria, o sulfonamides are attached to the dihydropteroate diphosphate in the place of the normal PABA giving false metabolite which is not capable of undergoing condensation with glutamic acid and inhibit the enzyme and the net result is inability of the bacteria to multiply as soon as the preformed folic acid in their cells is used up and further nucleic acid biosynthesis becomes impossible. o Bacteria which are able to take up preformed folic acid into their cells are resistant to sulfonamides. Structure-activity Relationships The strongly electron withdrawing character of the aromaticSO2 group makes the nitrogen atom to which it is directly attached partially electropositive, thus increasing the acidity of the hydrogen atoms attached to the nitrogen so that this functional group is slightly acidic Replacement of one of the NH2 hydrogen by an electron withdrawing heteroaromatic ring was not only consistent with antimicrobial activity but also greatly acidified the remaining hydrogen and dramatically enhanced potency and dramatically increases the water solubility under physiologic conditions. The poor water solubility of the earliest sulfonamides led to occasional crystallization in the urine (crystalluria) and resulted in kidney damage because the molecules were unionized at urine pH values. Structure-activity Relationships Therapeutic Applications Sulfisoxazole and its pro-drug acetyl sulfisoxazole Its clinical use is restricted to the treatment of the primary uncomplicated urinary tract infections. Sulfisoxazole is well absorbed following oral administration distributes widely and is excreted by the kidneys. Therapeutic Applications Sulfonamides are deactivated by acetylation at N-4 and glucuronation of the aniline nitrogen in the liver. Allergic reactions are the most common and take the form of rash, photosensitivity and drug fever. The most severe side effect is the Stevens-Johnson syndrome characterized by sometimes-fatal erythrema multiforme and ulceration of mucous membranes of the eye, mouth and urethra. Therapeutic Applications o Other sulfonamides still in use sulfamethizole and sulfamethoxazole. include sulfadiazine, Therapeutic Applications o Multiple (or triple) sulfas are a 1:1:1 combination of sulfabenzamide, sulfacetamide and sulfathiazole which used as a cream for carderella vaginalis vaginal infection Therapeutic Applications o Sulfasalazine is a pro-drug given orally and is largely not absorbed in the gut so the majority of the dose is delivered to the distal bowel where reductive metabolism by gut bacteria converts the drug to sulphapyridine and 5-aminosaliclic acid (Mesalamine). Therapeutic Applications o The liberation mesalamine, an anti-inflammatory agent, is the purpose for administering this drug. This agent is used to treat ulcerative colitis and Crohns disease. Direct ad- ministration of salicylates is otherwise irritating to the gastric mucosa. Mechanism of Action Trimethoprim inhibits the dihydrofolate reductase required for reduction of the exogenous folic acid stepwise to dihydrofolic acid and then to tetrahydrofolic acid an important cofactor essential for purine biosynthesis and ultimately for DNA synthesis. Endogenous produced dihydrofolate must also reduced by the same enzyme to enter the pathway involved in DNA synthesis. The bacterial enzyme and the mammalian enzyme both efficiently catalyze the conversion of dihydrofolic acid to tetrahydrofolic acid, but the bacterial enzyme is sensitive to inhibition by trimethoprim by up to 40,000 times lower concentrations than is the mammalian enzyme. This difference explains the useful selective toxicity of trimethoprim Trimethopim 5-[3,4,5-Trimethoxhyphenyl)methyl]-2,4-pyrimidinediamine H3CO H2N N H3CO NH2 N H3CO Synthesis H3CO H3CO O H3CO H + CN CN C2H5O H3CO CH2OC2H5 H3CO H3CO H2N H3CO NH H3CO NH H2N H2N N H3CO H3CO NH N H H3CO NH2 N NH H3CO Mechanism of Action Therapeutic Application Trimethoprim is used as a single agent for the oral treatment of uncomplicated urinary tract infections caused by with the susceptible bacteria Most commonly used in 1:5 fixed ratio sulfamethoxazole (Bactrim, Septra). This combination is not only synergistic but is less likely to induce bacterial resistance than either agent alone. These agents block sequentially at two different steps in the same essential pathway, and this combination is extremely difficult for a naive microorganism to survive. Combined with sulfamethoxazole, it is used for oral treatment of urinary tract infections, shigellosis, otitis media, traveler's diarrhea, and bronchitis. The most frequent side effects of are rush, nausea and vomiting. Quinolones The quinolone antimicrobials comprise a group of synthetic substances possessing in common an N-1-alkylated 3-carboxy pyrid-4-one ring fused to another aromatic ring, which itself carries other substituents. Nalidixic acid and cinoxacin are classified as first generation quinolones based on their spectrum of activity and pharmacokinetic properiteis. They are considered minor urinary tract disinfectants that are primarily effective against Gram (-ve) bacteria. Quinolones Quinolones were of little clinical significance until the discovery that the addition of a fluoro group at the 6-position of the basic nucleus greatly increased the biological activity. Norfloxacin, the first of the second generation quinolones, has a broad spectrum of activity and equivalent in potency to many of the fermentation compounds. Quinolones Following Norfloxacin introduction, more than a thousand second-, third-, and fourth generation analogues have introduced. They include ofloxacin, alatrofloxacin, levofloxacin, norfloxacin, etc. norfloxacin, sparofloxacin, ciprofloxacin, trovofloxacin, O F Ciprofloxacin 1-Cyclopropyl-6-fluoro-1,4-dihydro-4-oxo7-(1-piperazinyl)-3-quinolinecarboxylic acid COOH N N HN Synthesis O O F F COOC2H5 O F COOC2H5 CH OC2H5 NH2 F F F OC2H5 O O F COOC2H5 F COOC2H5 HN Cyclization F F N H F O F N HN COOH N NH hydrolysis N Quinolones Mechanism of Action The quinolones are rapidly bactericidal largely as a consequence of inhibition of DNA gyrase and topoisomerase IV key bacterial enzymes that dictate the conformation of DNA so that it can be stored properly, unwound, replicated, repaired, and transcribed on demand. Inhibition of DNA gyrase and topoisomerase I+V makes a cell’s DNA inaccessible and leads to cell death. Humans shape their DNA with a topoisomerase II which does not bind quinolones at normally achievable doses so the quinolones do not kill host cells Structure-Activity relationship The carboxy-4-pyridone nucleus is essential for activity The carboxylic acid and the ketone are involved in binding to the DNA/DNA-gyrase enzyme system. Reduction of the 2,3-doublc bond or the 4-keto group inactivates the molecule, and substitution at C-2 interferes with enzyme-substrate complexation. Fluoro substitution at the C-6 greatly improves antimicrobial activity by increasing the lipophilicity of the molecule, which in turn improves the drugs penetration through the bacterial cell wall. Additionally, C-6 fluoro increases the DNA gyrase inhibitory action Structure-Activity relationship Alkyl substitution on the piperazine (lomefloxacin and ofloxacin) decrease binding to GABA, as does the addition of bulky groups at the N-1 position (sparfloxacin). The cyclopropyl substitution at N-l broads activity of the quinolones to include activity against atypical bacteria. The introduction of a third ring to the quinolones nucleus gives rise to ofloxacin which has an asymmetric carbon at the C-3 position. The S(-)-isomer (levofloxacin) is twice as active as ofloxacin and 8- to 128fold more potent than the R(+)-isomer resulting from increased binding to the DNA-gyrase. Structure-Activity relationship Several of the quinolones produce mild to severe photosensitivity. A C-8 halogen appears to produce the highest incidence of photosensitivity via singlet oxygen and radical induction. Lomefloxacin has been reported to have the highest potential for producing phototoxicity. Substitution of a methoxy group at: C-8 has been reported to reduce the photosensitivity (gatilfoxacin). Chemical Incompatabilities The quinolones chelate polyvalent metal ions (Ca2+ Mg+2, Al+3, and Fe+2) to form less water-soluble complexes and thereby lose considerable potency. Thus co-administration of certain antacids, hematinics, tonics and consumption of dairy products soon after quinolone administration is contraindicated Side Effects Among the toxicities associated with quinolones is a proconvulsant action, especially in epileptics which mainly associated with the first generation agents. Other CNS problems include hallucinations, insomnia, visual disturbances. Some patients also experience diarrhea, vomiting, abdominal pain and anorexia. Fluoroquinolones drugs are generally much better tolerated. Therapeutic Applications The second-generation quinolones are more widely used than the first. Norfloxacin is mainly used for urinary tract infections The others, particularly ciprofloxacin, are also used for prostatitis, upper respiratory tract infections, bone infections, septicemia, staphylococcal and pseudomonal endocarditis, meningitis, sexually transmitted diseases chronic ear infections, and purulent osteoarthritis. Lomefloxacin is used once daily for urinary tract and upper respiratory tract microorganisms infections due to susceptible Metronidazole Initially introduced for the treatment of vaginal infection caused by amoeba, it is also useful for the treatment of trichomoniasis, giardiasis and Gardnerella vaginalis infections. Metronidazole is also a component of a multidrug cocktail used to treat Helicobacter pylori infections associated with gastric ulcers. Metronidazole use is associated with allergic rashes and CNS disturbances, including convulsions in some patients. Nitrofurans (e.g. Nitrofurantoin) Nitrofurantoin a widely used oral antibacterial for prophylaxis or treatment of urinary tract infections when kidney function is not impaired, and it inhibits kidney stone growth. Nausea and vomiting are common side effects. This is avoided in part by slowing the rate of absorption of the drug through use of wax- coated large particles (Macrodantin). Nitrofurantoin inhibits DNA and RNA functions Methenamine A venerable drug used for the disinfection of acidic urine Structurally methenamine is a low molecular weight polymer of ammonia and formaldehyde which reverts to its components under mildly acid conditions. Formaldehyde is the active antimicrobial component. Methenamine is used for recurrent urinary tract infections Phosphomycin Phosphomycin inhibits enolpyruvial transferase (an enzyme catalyzing an early step in bacterial cell wall biosynthesis) resulting in reduced synthesis of peptidoglycan an important component in the bacterial cell wall. Phosphomycin is bactericidal against E. coli infections. Dapsone (DDS) Mechanism of Action Dapsone, a bacteriostatic agent, act through competitive inhibition of p-aminobenzoic acid incorporation into folic acid ,it is used for treatment of leprosy. Dapsone (DDS) Structure-activity Relationship Isosteric replacement of one benzene ring resulted in the formation of thiazolsulfone. Although still active, it is less effective than DDS. Substitution on the aromatic ring, to produce acetosulfone, reduces activity while increasing water solubility and decreasing GI irritation. Dapsone (DDS) Structure-activity Relationship Adding methanesulfinate to DDS to give the water soluble sulfoxone sodium which is hydrolyzed in vivo to produce DDS. Sulfoxone sodium is used in individuals who are unable to tolerate DDS due to GI irritation, but it must be used in a dose three times that of DDS because of inefficient metabolism to DDS. Dapsone (DDS) Metabolism The major metabolic product of DDS results from Nacetylation in the liver by N-acetyltransferase