* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Bioavailability & Bioequivalence
Tablet (pharmacy) wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Compounding wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Drug discovery wikipedia , lookup
Prescription costs wikipedia , lookup
Plateau principle wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Drug design wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Drug interaction wikipedia , lookup
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence:
Pharmacokinetic Principles
Sandip K. Roy, Ph.D.
Exploratory Clinical Development – PK
Novartis Pharmaceutical Corporation
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
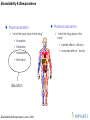
Pharmacokinetics
“what the body does to the drug”
Absorption
Distribution
Metabolism
Elimination
disposition
Bioavailability & Bioequivalence, June 2, 2004
Pharmacodynamics
“what the drug does to the
body”
wanted effects - efficacy
unwanted effects - toxicity
Bioavailability & Bioequivalence
Pharmacokinetics
Dose regimen
Bioavailability & Bioequivalence, June 2, 2004
Exposure
Pharmacodynamics
Site of action
Response
Bioavailability & Bioequivalence
Pharmacokinetics is either directly or
indirectly associated with just about
every part of pharmaceutical business:
Research and the selection of a promising molecule
Dosage formulation development
Dose regimen
Toxicology and safety assessment
Dosing recommendations for age groups & subpopulations (renal/hepatic/race/DDI)
Effect of meals and dosing
Marketing claims and promotion
Generic substitution
Manufacturing changes
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
General Outline
Basic Pharmacokinetic Concepts
Bioavailability
Definition
How absorption affects bioavailability?
Food Effect
How drug metabolism affects bioavailability?
How transporters affect bioavailability?
Bioequivalence
Definition
Bio-IND
Waivers of In Vivo Study Requirements
Biopharmaceutics Classification System (BCS)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Basic Concepts
Easy to understand using intravenous route
Drug Product
No absorption phase
Simple to follow
Concepts clear with less assumptions
Drug in
Blood
Excretion
Distribution to
Tissue and Receptor sites
Metabolism
Need some math background
algebra, log scale, Simple linear
Equations etc
complex math (differential equations,
statistical concepts etc) for Modeling,
Population PK, PK-PD etc.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
IV administration, contd.,
Following dose administration, we
need to follow its drug’s disposition
to understand its PK characteristics.
This is achieved by analyzing the
changes of the drug and/or its
metabolite(s) in blood, plasma,
urine etc.
A simple approach is to generate
Drug Concentration-Time profile
Dosing
Sampling at
Pre-determined
Time intervals
Bioavailability & Bioequivalence, June 2, 2004
Blood withdrawal
Bio-analytics
Conc. vs time
profiles
Bioavailability & Bioequivalence
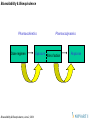
Concentration versus Time Profiles
Broadly the concentration – time profiles can be viewed as two different ways
One-Compartment Model
Dose
1
Assumes body as one compartment
k
Two-Compartment Model
Central compartment (drug entry and
elimination)
Dose
2
1
Tissue compartment (drug distributes)
k
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
The one compartment model linear
assumes that the drug in question is
evenly distributed throughout the
body into a single compartment.
This model is only appropriate for
drugs which rapidly and readily
distribute between the plasma and
other body tissues.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
The distribution phase for aminoglycosides is only 15-30
minutes, therefore, we can use a one-compartment
model with a high degree of accuracy
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Drugs which exhibit a slow
equilibration with peripheral
tissues, are best described with
a two compartment model
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Vancomycin is the classic example, it's distribution phase is 1 to 2
hours. Therefore, the serum level time curve of vancomycin may
be more accurately represented by a 2-compartment model.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Volume of Distribution
The concentration in plasma is achieved after distribution is
complete is a function of dose and extent of distribution of drug
into tissues
This extent of distribution can be determined by relating the
concentration obtained with a known amount of drug in the
body
Concentration is related to the amount by a constant, VOLUME
(V)
Amount (mg) = C (mg/L) * V (L)
OR
V = Amount / C
V is known as Apparent Volume of distribution.
Plasma volume ~ 3 L; Extracellular water ~16 L; Total body water ~ 42 L
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Volume of Distribution
Case -1
At Time zero, the drug amount in the body is the dose
(500 mg)
Calculated drug concentration at Time zero is 50 mg/L
Then, the V = 10 L
Case -2
Dose = 500 mg
Calculated Concentration at time Zero is 5 mg/L
Then, V = 100 L
Examples: Ibuprofen: V is 10 L; Diovan 17 L; Digoxin: ~500L;
Chloroquin: 15000 L
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Concentration (Units/ml)
Area Under the Concentration – Time Curve (AUC)
A quantitative measure for exposure
from dosing time to time ‘t’
10
An important parameter in PK
AUC(t) and AUC(inf)
1
Determined by trapezoidal method
0.1
AUC(inf) = AUC(t) + Ct/k
Units: Conc*t (mg/L * h)
0.01
0
5
10
15
Time (hr)
20
Proportional to Dose (linear PK)
Accuracy of the estimate depends
on frequency of sampling
Area Under the Curve (AUC)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
How is drug excreted/eliminated?
The Kidneys
This is the main excretory organ for drugs
The Nephron: Glomerulus, proximal tubule, loop of Henle,
distal tubule, and collecting tubule
Drug enters the lumen of the nephron by filtration and
secretion
Filtration occurs in the glomerulus; secretion is primarily
restricted to the proximal tubules
Reabsorption occurs all along the nephron; Active
reabsorption usually occurs in the proximal tubule
Appearance of drug in the urine is the net result of
filtration, secretion, and reabsorption
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Drug metabolism/biotransformation
This mainly occurs in the liver, via liver enzymes.
But it can also occur in the blood plasma or at
various other places (stomach, intestines, lungs,
skin, or kidneys) directly by various enzymes at
those locations
In any case, these metabolites are then
excreted/eliminated (more easily than would the
parent molecule have been) metabolites are
often smaller in size, ionized
Some drugs are excreted/eliminated in
unmetabolized form, as the original drug
molecule (e.g. Lithium)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Other Routes of Excretion/Elimination
In bile (which then empties into gut,
excreted in feces)
[can excrete from 5 to 95% of drug dose, esp. antibiotics]
In sweat, saliva, tears, exhaled breath, milk,
hair, nails
[as heart rate increases --- pulmonary circulation --- which
then increases amounts of breath exhaled --- more drug
eliminated]
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Concept of “Half Life”
½ life = how much time it takes for blood levels of drug to
decrease to half of what it was at equilibrium
There are really two kinds of ½ life…
“distribution” ½ life = when plasma levels fall to half what
they were at equilibrium due to distribution to/storage in
body’s tissue reservoirs
“elimination” ½ life = when plasma levels fall to half what
they were at equilibrium due to drug being metabolized
and eliminated
It is usually the elimination ½ life that is used to determine
dosing schedules, to decide when it is safe to put patients on
a new drug
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Concept of “Half Life”
5
Conc. [mg/L]
4
3
2
1
0
0
4
8
12
Time [hours]
Bioavailability & Bioequivalence, June 2, 2004
16
20
24
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Elimination
ke
lny ln
y
2
T1/2
lny lny ln2
ke
T1/2
ln2
ke
T1/2
0.693
ke
T1/2
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
“Rule of Five”
5x the elimination ½ life = time at which the
drug is “completely” (97%) eliminated from the
body
1x ½ life - 50% of the original drug removed
2x ½ life - 75%
3x ½ life - 87.5%
4x ½ life - 93.75%
5x ½ life - 96.875%
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Clearance
“Of the concepts in pharmacokinetics, clearance has the greatest
potential for clinical applications.
It is also the most useful parameter for the evaluation of an
elimination mechanism.”
Rowland & Tozer
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Clearance
Quantifies Elimination
Is the volume of body fluid cleared per
time unit (L/h, mL/min)
Is Usually Constant
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Clearance
Proportionality factor relating rate of drug elimination to
plasma drug concentration
Rate of eliminatio n CL C
Rate of eliminatio n
CL
C
(dx/dt)
CL
C
Integrate
DoseIV
CL
AUC
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Clearance
Rate of elimination is proportional to the amount (A) of
drug present
Rate of eliminatio n k * A
Rate of eliminatio n k * V * C
Rate of eliminatio n
k * V
C
Clearance k * V
0.693 * V
Clearance
Half - life
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Dependence of Half-life on
Clearance and Volume
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Why is Clearance Important?
Clearance is the one parameter that
determines the maintenance dose rate
required to achieve a desired plasma conc.
Dosing rate = clearance X desired plasma
conc.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
For a given dose rate, the blood drug concentration is
inversely proportional to clearance
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
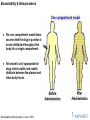
Concentration
Multiple Dose Administration
Time (hr)
Minimum and maximum conc should be within therapeutic window –
depends on dose, frequency and t1/2
Depending on dosing frequency and t1/2, accumulation occurs
Degree of accumulation is important for safety assessment purposes
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability and Its Assessment
Bioavailability:
The rate and extent to which the parent
compound reaches the general circulation.
Absolute Bioavailability
requires I.V. administration
Ratio of the oral:intravenous AUC values normalized for dose
Fabs= (AUC oral / AUC iv)*(Dose iv / Dose oral)
Relative Bioavailability
no I.V. reference
comparison AUC values (ratio) of different dosage forms /
formulations
Frel = (AUC a / AUC b) * (Dose b /Dose a)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
5
5
4
4
Conc. [mg/L]
Conc. [mg/L]
Solution
Capsule
3
2
1
3
2
1
0
0
0
4
8
12
16
20
24
Time [hours]
20 mg administered as an
i.v. bolus (Diovan)
Bioavailability & Bioequivalence, June 2, 2004
0
4
8
12
16
20
24
Time [hours]
80 mg given as a solution and
a capsule (Diovan)
Bioavailability & Bioequivalence
F=0.6
Solution
Capsule
80
F=0.4*
16
14
60
F=0.2*
12
10
40
AUC
% of f remaining to be absorbed
100
20
8
6
4
2
0
0
2
4
6
8
Time [hours]
10
0
I.v. (20 mg)
P.O. (80 mg) P.O. (80 mg)
Capsule
Solution
*dose - adjusted
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
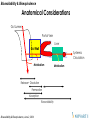
Anatomical Considerations
Gut Lumen
Portal Vein
Liver
Gut Wall
Systemic
Circulation
Metabolism
Metabolism
Release + Dissolution
Permeation
Elimination
Absorption
Bioavailability
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
How Absorption
affects Bioavailability?
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Absorption
Absorption is defined as the
process by which a drug
proceeds from the site of
administration to the site of
measurement.
Drugs are frequently
administered extravascularly
oral, sublingual
intramuscular,
topical, patches, inhalation
Absorption is a prerequisite for a
drug to exert it’s pharmacologic
effect (other than local effect)
Several possible sites contribute
to the loss
Bioavailability & Bioequivalence, June 2, 2004
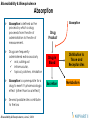
Absorption
Drug
Product
Drug in
Blood
Excretion
Distribution to
Tissue and
Receptor sites
Metabolism
Bioavailability & Bioequivalence
Plasma Concentration-Time Profile for a Drug Following a
Single Oral Dose
Rate of drug accumulation at
any time:
dDBODY/dt= dDABS/dt - dDELIM/dt
Absorption Phase:
dDABS/dt > dDELIM/dt
At time of peak drug conc.:
dDABS/dt = dDELIM/dt
Post-absorption Phase:
dDABS/dt < dDELIM/dt
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
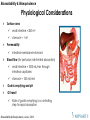
Physiological Considerations
Surface area
small intestine = 200 m2
stomach = 1 m2
Permeability
intestinal membrane>stomach
Blood flow (for perfusion rate-limited absorption)
small intestine = 1000 mL/min through
intestinal capillaries
stomach = 150 mL/min
Gastric emptying and pH
GI transit
Rate of gastric emptying is a controlling
step for rapid absorption
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Physico-Chemical Factors
Partition Theory
Ionization, pH-pKa Relationship
Polymorphism
Particle Size
Complexation
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
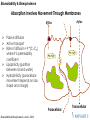
Absorption Involves Movement Through Membranes
Efflux
Influx
Passive diffusion
Active transport
Rate of diffusion = P *(C1-C2)
where P is permeability
coefficient
Lipophilicity (partition
between oil and water)
Hydrophilicity (paracellular
movement depends on size,
shape and charge)
Paracellular
Bioavailability & Bioequivalence, June 2, 2004
Transcellular
Bioavailability & Bioequivalence
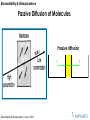
Passive Diffusion of Molecules
Passive diffusion
1
Bioavailability & Bioequivalence, June 2, 2004
2
Bioavailability & Bioequivalence
Comparison of the Rates of Drug Absorption
A = Passive diffusion
B = Active transport/
carrier mediated
system
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Percent Dose Absorbed vs. Human Permeability
Very low concentration
No saturation effects
Already in solution
No dissolution effects
Percent Absorbed (%)
Propanolol
Piroxicam
Naproxen
Ketoprofen
L-leucine
100
Phenylalanine
75
Benserazide
L-Dopa
Metoprolol
50
D-glucose
Antipyrine
Terbutaline
Furosemide
25
Atenolol
Enalaprilate
0
0
2
4
6
8
Human Permeability (104, cm/sec)
Bioavailability & Bioequivalence, June 2, 2004
10
Bioavailability & Bioequivalence
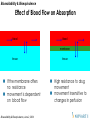
Effect of Blood Flow on Absorption
blood
blood
membrane
tissue
If the membrane offers
no resistance
movement is dependent
on blood flow
Bioavailability & Bioequivalence, June 2, 2004
tissue
High resistance to drug
movement
movement insensitive to
changes in perfusion
Bioavailability & Bioequivalence
pH – pKa Ionization
Weak acid
pka - pH = log [(un-ionized)/(ionized)]
Weak base
pka - pH = log [(ionized)/(un-ionized)]
Examples:
Aspirin, pka : 3.5, at pH = 1, mostly unionized
Phenytoin, pka : 8.3, unionized in stomach
Diazepam, pka : 3.3, mostly ionized in stomach
Procainamide, pka : 9.5, mostly ionized in stomach
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Gastrointestinal pH and Transit Time in the
Fasted State
Region
pH
Residence
time
Stomach
1.5-2
0-3 hours
Duodenum
4.9-6.4
3-4 hours
Jejunum
4.4-6.4
3-4 hours
Illeum
6.5-7.4
3-4 hours
Colon
7.4
Up to 18 hours
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Assessment of Drug Absorption
Absorption is measured as Rate of Absorption, ka. and
Extent (AUC)
For Rate - Need to fit the data and it is model
dependent
A surrogate is Cmax/AUC
Example:
Lescol capsule (IR) :
0.37 hr-1
Lescol XL:
0.19 hr-1
Usually (also) measured as Cmax and Tmax
Cmax
Tmax
Lescol IR
438
0.5-1 h
Lescol XL
101
1.5-4 h
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Effect of a Change in Absorption Rate Constant (Ka) on
Plasma Drug Concentration Versus Time Curve
0.5/hr
0.2/hr
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Interactions in Oral Drug Absorption
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Pharmacokinetic Assessment of Absorption
Interactions
Clinically significant interactions are
typically assessed in terms of:
Rate of Absorption:
peak plasma drug concentrations (Cmax)
time to Cmax (tmax)
Extent of Absorption:
area under the concentration-time curve
(AUC)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Effect of Absorption Interactions on Drug Plasma
Concentration Profiles
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Effect of Food
A required study – helps for dosage
administration in Clinical Trials
Measure PK parameters (rate and
extent) under Fasted and Fed conditions.
Single dose cross over study is
recommended.
FDA Guidance gives type of food
High Fat Meal (breakfast) – total of
800 – 1000 calories
of which 150 cal from Proteins, 250 cal
from carbohydrates and 500 – 600 cal
from fat.
Bioavailability & Bioequivalence, June 2, 2004
Test Meal
2 eggs fried in butter
2 strips of bacon
2 slices of toast with butter
4 oz of hash-brown potatoes
8 oz of whole milk
Bioavailability & Bioequivalence
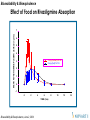
Effect of Food on Rivastigmine Absorption
MEAN RIVASTIGMINE PLASMA LEVELS (ng/mL)
7
6
5
4
3 mg (fasted) N=20
3 mg (fed) N=19
3
2
1
0
-1
0
2
4
6
TIME (hrs)
Bioavailability & Bioequivalence, June 2, 2004
8
10
12
14
Bioavailability & Bioequivalence
Concentration (ng/mL)
Effect of Food on Lescol XL
150
Fasted
Fed
120
90
60
30
0
0
6
12
Tim e (h)
Bioavailability & Bioequivalence, June 2, 2004
18
24
Bioavailability & Bioequivalence
Food Effect
Statistical analysis is done for significant
difference
PK data interpretations are made in conjunction
with clinical experience / clinical significance
Attention should be paid for the absorption rate
and total exposure with and without food. Cases
when time to peak concentration is important
(analgesic)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Summary
Absorption is influenced by physico-chemical properties of
the drug, formulation factors, and the anatomy and
physiologic functions at the site of drug absorption.
Drug absorption process may be zero order (active transport)
or first order (passive diffusion) process.
Highly soluble and highly permeable drugs are rapidly
absorbed.
Estimation of drug absorption and bioavailability is critical in
early stage drug development.
BE studies are required for changing formulations etc.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
How Drug Metabolism
affects Bioavailability?
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Drug metabolism/Biotransformation
Liver is the main site of drug metabolism
Extrahepatic:
Gut wall
Intestinal Flora
Lung
Kidney
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Reactions Catalyzed by Drug metabolizing
enzymes
Oxidative reactions (Phase I)
dealkylation
hydroxylation
oxidation
Deamination
Conjugation reactions (Phase II)
glucuronidation
glutathione conjugation
sulfation
acetylation
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
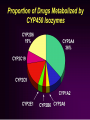
How Drug Metabolism Affects Bioavailability?
Genetic (polymorphism in expression of
enzymes in a population)
CYP2D6, CYP2C19, NAT2, etc.
Environmental (food, smoking)
Grapefruit juice (↑AUC and ↑Cmax)
Drug-Drug interaction
Inhibition (↑AUC and ↑Cmax)
Induction (↓AUC and ↓Cmax)
Age
Disease (hepatic impairment)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
St. John’s Wort
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
How Transporters
affect Bioavailability?
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Energy Dependent Efflux Transporters –
ATP-binding cassette (ABC) proteins
Work against concentration gradient
MDR1 (P-glycoprotein)
MDR3
MRP2 (multidrug resistance associated
protein, cMOAT)
BSEP (bile salt export pump)
BCRP (breast cancer resistance protein)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
How Transporters Affect Bioavailability?
P-glycoproteins expressed in
Intestine limit absorption low BA
liver increase bile secretion low BA
kidney increase secretion in urine
shorten t1/2
Brain protect CNS from penetration of
toxic drugs or decrease efficacy of CNS
drugs
Some lymphocytes drug resistance for HIV
drugs
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioequivalence: Background
Using bioequivalence as the basis for approving generic copies
of drug products was established by the “Drug Price
Competition and Patent Term Restoration Act of 1984,” also
known as the Waxman-Hatch Act.
This Act expedites the availability of less costly generic drugs by
permitting FDA to approve applications to market generic
versions of brand-name drugs without conducting costly and
duplicative clinical trials.
At the same time, the brand-name companies can apply for up
to five additional years longer patent protection for the new
medicines they developed to make up for time lost while their
products were going through FDA's approval process. Brandname drugs are subject to the same bioequivalence tests as
generics upon reformulation.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioequivalence
Definition - CFR 320.1
It is the absence of significance difference in the
rate and extent to which active ingredient or
active moiety in pharmaceutical equivalent or
pharmaceutical alternative becomes available at
the site of drug action when administered at the
same molar dose under similar conditions in an
appropriately designed study
Note: BE has a specific definition and regulatory
requirements. BE is not the same as the BA
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
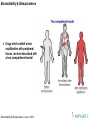
When do we do BE studies ?
Clinical Service Form to Final Market Form
Change of formulations (capsules to
tablet)
Generic Formulations
Change of Process or manufacturing site
(some times)
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioequivalence
Test Batch Size: 100,000 units or 10% of
Production size whichever is greater
Retention Samples: Need to retain samples
at the study site for further analysis (5 times).
Most of the BE studies are audited by HAs
especially for NMEs
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bio-IND
“The primary purpose of a Bio-IND is to ensure that
the proposed product is safe for use in human test
subjects and does not expose them to undue risk
and untoward effects from the drug product”
MAPP 5240.4, CDER, FDA
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Contents of a Bio-IND
OGD's new policy is that in addition to a protocol,
sufficient information must be submitted in a Bio-IND to
enable an OGD bioequivalence reviewer and a
review chemist to determine the safety of the
formulation to be used in the proposed
bioequivalence study.
Only one protocol per Bio-IND submission
Components and composition of the generic drug to
be used in the bioequivalence study including the
amounts of the active ingredient(s) and excipients
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Contents of a Bio-IND
Tests and specifications for identity, strength, quality, and
purity for active ingredient(s) and Certificates of Analysis of
excipients;
Method and place of manufacturing including the type of
equipment, batch size and batch records
Tests and specifications for the finished dosage form
(Certificates of Analysis);
Stability testing data on the drug product stored for three
months at 400C and 75% relative humidity including
information on the container/closure system(s) used in the
stability tests unless other conditions are appropriate for that
product.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Filing and Review Procedures
A Bio-IND received in the Document Room will be identified
by its cover letter and standard form 1571.
The Bio-IND will then be routed to the central CSO staff
which will review the submission for acceptability and send
out an acknowledgment letter under the signature of the
Director, OGD.
If the Bio-IND does not contain the information described in
POLICY AND PROCEDURE, Contents of a Bio-IND, a refuse to
file letter will be issued and the firm will have to correct the
deficiencies and resubmit the Bio-IND.
If a Bio-IND is determined to be acceptable for filing, the
thirty-day safety review clock will start on the date of
receipt of the submission.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Filing and Review Procedures
The central CSO staff will send
one copy to the appropriate OGD Chemistry Branch
based upon the pharmacological class of the drug to be
studied
another copy to the Division of Bioequivalence or the
appropriate NDE reviewing Division, and
a third copy to the Document Room to be filed
Normally, the Division of Bioequivalence will review the
protocol for the bioequivalence study to ensure that
the safety of subjects entering the study will not be
compromised.
If a protocol raises a medical issue such as proposing
to administer a dose not addressed in the labeling, a
medical officer in NDE will be consulted.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Filing and Review Procedures
Information on chemistry, manufacturing, and controls
will be reviewed by one of the two Divisions of Chemistry
to ensure the safety of the study volunteers. A more
detailed review will be conducted of the chemistry,
manufacturing and controls information that is later
submitted in the ANDA.
A CSO will be assigned the responsibility to track the BioIND through the review process, including checking
periodically with the reviewing divisions on the status of
the reviews.
If the CSO determines that the safety reviews will not be
completed within thirty days, he or she will inform the firm
and may request that the start of the study be deferred
until the reviews are completed.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Filing and Review Procedures
Upon completion of the safety reviews, OGD will notify the
firm that the study may begin or that the study has been
placed under a clinical hold pursuant to 21 CFR § 312.42.
The chemistry and bioequivalence reviews of the Bio-IND,
when completed, will be sent back to the central CSO
staff. That staff will prepare the appropriate action letter for
the signature of the Director, OGD.
A bioequivalence study completed under a Bio-IND should
be submitted in the ANDA which it supports. No
bioequivalence studies should be submitted as
amendments to Bio-IND's.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Waivers of In Vivo Study
Requirements
Criteria (21 CFR 320.22)
In vivo bioequivalence is self evident
Parenteral Solutions
Inhalation anesthetics
Topical skin solutions
Oral solutions
Different proportional strength of product with
demonstrated BE
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
The Biopharmaceutics Classification
System (BCS) Guidance
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Purpose of the BCS Guidance:
Expands the regulatory application of the BCS and
recommends methods for classifying drugs.
Explains when a waiver for in vivo bioavailability and
bioequivalence studies may be requested based on
the approach of BCS.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Classifications
According to the BCS, drug substances are
classified as follows:
Class I - High Permeability, High Solubility
Class II - High Permeability, Low Solubility
Class III - Low Permeability, High Solubility
Class IV - Low Permeability, Low Solubility
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
CLASS BOUNDARIES
A drug substance is considered HIGHLY SOLUBLE when
the highest dose strength is soluble in < 250 ml water
over a pH range of 1 to 7.5.
A drug substance is considered HIGHLY PERMEABLE
when the extent of absorption in humans is
determined to be > 90% of an administered dose,
based on mass-balance or in comparison to an
intravenous reference dose.
A drug product is considered to be RAPIDLY
DISSOLVING when > 85% of the labeled amount of
drug substance dissolves within 30 minutes using USP
apparatus I or II in a volume of < 900 ml buffer
solutions.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
SOLUBILITY DETERMINATION
pH-solubility profile of test drug in aqueous
media with a pH range of 1 to 7.5.
Shake-flask or titration method.
Analysis by a validated stability-indicating
assay.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
PERMEABILITY DETERMINATION
Extent of absorption in humans:
Mass-balance pharmacokinetic studies.
Absolute bioavailability studies.
Intestinal permeability methods:
In vivo intestinal perfusions studies in humans.
In vivo or in situ intestinal perfusion studies in animals.
In vitro permeation experiments with excised human or
animal intestinal tissue.
In vitro permeation experiments across epithelial cell
monolayers.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Determining Drug Product Dissolution Characteristics and
Dissolution Profile Similarity
Dissolution testing should be carried out in USP
Apparatus I at 100 rpm or Apparatus II at 50 rpm
using 900 ml of the following dissolution media:
0.1N HCl or Simulated Gastric Fluid USP without enzymes
a pH 4.5 buffer
a pH 6.8 buffer or Simulated Intestinal Fluid USP without
enzymes
For capsules and tablets with gelatin coating
Simulated Gastric and Intestinal Fluids USP (with
enzymes) can be used.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Dissolution Profile Similarity
A minimum of 12 dosage units of a drug product should be
evaluated to support a biowaiver request.
Samples should be collected at a sufficient number of intervals to
characterize the dissolution profile of the drug product (e.g., 10,
15, 20, and 30 minutes).
When comparing the test and reference products, dissolution
profiles should be compared using a similarity factor (f2). The
similarity factor is a logarithmic reciprocal square root
transformation of the sum of squared error and is a measurement
of the similarity in the percent (%) of dissolution between the two
curves.
f2 = 50 * log {[1+(1/n)*t=1n (Rt - Tt)2]-0.5 * 100}
Two dissolution profiles are considered similar when the f2 value is
50.
Note: When both test and reference products dissolve 85% or more of the label
amount of the drug in 15 minutes using all three dissolution media recommended
above, the profile comparison with an f2 test is unnecessary.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Conditions for BCS Bio-waivers
Firms can request waivers of in vivo
testing for Class 1 drug substances
Drug products must meet these criteria:
Immediate-release solid oral dosage forms
Highly soluble, highly permeable drug substance
Rapid in vitro dissolution
Note: Waivers not applicable for narrow therapeutic
range therapeutic range (Digoxin, Lithium, phenytoin,
warfarin) drugs
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Class I: Dissolution
USP Apparatus I (100 rpm) or II (50 rpm)
Three media
0.1 N HCl or SGF USP without enzymes 0.1 N HCl or SGF USP
without enzymes
pH 4.5 buffer pH 4.5 buffer
pH 6.8 buffer or SIF USP without enzymes
NLT 85% dissolves within 30 minutes
Similarity factor (f2) for test (T) v. reference (R) profile
comparisons should > 50
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Class I: Solubility
Highest dose strength should be soluble in <
250 mL
Volume is derived from BE protocols
Doses are generally administered with about 8 oz water
Determinations should use a range of pH
values over 1 to 7.5, a temperature of 370C,
and equilibrium conditions
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Class I: Permeability
In vivo methods include determination absolute
BA (> 90%) or mass balance
In vitro intestinal permeability can be
determined by several methods
One method is use of cultured epithelial cell monolayers
A single method may be sufficient
Stability in GI tract should be determined
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Class I: Permeability
For prodrugs, permeability depends on
mechanism, anatomical site of conversion
When conversion occurs prior to intestinal
permeation, measure permeability of active
moiety
When conversion occurs after intestinal
permeation, measure permeability of
prodrug
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
BCS Class I: Excipients
Quantity of excipients should be consistent with
intended function
Large quantities of some surfactants may be
problematic
polysorbate 80
Mannitol
sorbitol
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Recent Federal Register Notice
FDA is proposing to amend its regulations to require
an ANDA applicant to submit data from all
bioequivalence studies (BE studies)
In the past, ANDA applicants have not typically
submitted additional BE studies conducted on the
same drug product formulation, such as studies that
do not show that the product meets these criteria.
FDA is proposing this change because the data
from additional BE studies may be important in
determination of whether the proposed formulation
is bioequivalent to the RLD and are relevant to
evaluation of ANDAs in general.
In addition, such data will increase understanding of
how changes in components, composition, and
methods of manufacture may affect formulation
performance.
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
Bioavailability & Bioequivalence, June 2, 2004
Bioavailability & Bioequivalence
References
Clinical Pharmacokinetics: Concepts and
Application - 3rd Edition
By Malcolm Rowland & Thomas N. Tozer
http://www.fda.gov/cder/guidance/index.htm
http://www.access.gpo.gov/nara/cfr/waisidx_03/21
cfr320_03.html
Bioavailability & Bioequivalence, June 2, 2004