* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Objectives - American Society for Pain Management Nursing
Survey
Document related concepts
Psychopharmacology wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug interaction wikipedia , lookup
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Drug discovery wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Transcript
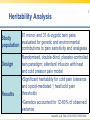
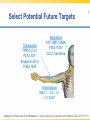
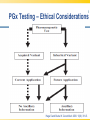
Opioid Pharmacogenomics: The Path to Personalized Medicine Leigh M. Boehmer, Pharm.D., BCOP Clinical Pharmacist, Medical Oncology Barnes Jewish Hospital March 8, 2014 Disclosure • Leigh M. Boehmer, Pharm.D., has no real or apparent conflicts of interest to report Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results Clinical Genetics Defined • Pharmacodynamics: drug activity at the target site/receptor • Pharmacokinetics: drug absorption, distribution, metabolism, elimination (ADME) • Pharmacogenomics (PGx): how variations in human genome affect drug response Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. Anticipated Benefits of Genomics • • • • • • Earlier detection of genetic predispositions Improved diagnostic accuracy and speed Gene therapies Decrease in healthcare costs Personalized treatment plans Rational drug design Available at ornl.gov/hgmis. Accessed on 12.28.13. Clinical Genetics Defined • Genotype: genetic makeup of an organism • Phenotype: physical characteristics of an organism • Epigenetics: phenotypes resulting from environment-caused chromosomal changes • Allele: one of two copies of a gene inherited from each parent • Mutation: any heritable change in a DNA sequence • Polymorphism: DNA sequence differences present in >1% of the population Available at ornl.gov/hgmis. Accessed on 12.28.13. Heritability Analysis 81 mono- and 31 di-zygotic twin pairs Study evaluated for genetic and environmental population contributions to pain sensitivity and analgesia Randomized, double-blind, placebo-controlled twin paradigm; alfentanil infusion with heat Design and cold pressor pain model •Significant heritability for cold pain tolerance and opioid-mediated ↑ heat/cold pain thresholds Results •Genetics accounted for 12-60% of observed variance Angst M, et al. Pain. 2012. 153(7):1397-1409. Pain Management Genetic Analysis Analgesic Polymorphic Genes Codeine CYP2D6 Fentanyl ABCB1, CYP3A4, CYP3A5, OPRM1 Oxycodone CYP2D6 Methadone ABCB1, CYP2B6, CYP3A4, CYP2D6 Morphine ABCB1, COMT, UGT2B7, OPRM1 Tramadol CYP2D6 ABCB1=ATP-binding cassette, subfamily B, member 1 OPRM1=mu-opioid receptor gene COMT=catechol-O-methyltransferase UGT=uridine 5’-diphosphate-glucuronosyltransferase Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. Opioid Metabolism: CYP450 variations • Phase I metabolism in liver – CYP2D6, CYP3A4, CYP2C19, and others • Spectrum of enzymatic activity – Poor, intermediate, extensive, ultra rapid • Tramadol to active metabolite via -2D6 • Codeine conversion to morphine via -2D6 • Oxycodone to active metabolite via -2D6 Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. Ma J, et al. Journal of Pharmacy Practice. 2012; 25(4): 417-27. CYP2D6 Phenotype Correlation Phenotype Frequency Phenotype CYP2D6 Variant Alleles Asians African Americans Caucasians Poor metabolizer *3, *4, *5, *6 1-2% 7% 7-10% Intermediate metabolizer *9, *10, *17, *29, *41 30% 30% 11% Extensive metabolizer *1 51.5% 37% 71% Ultra rapid metabolizer *1, *2 gene duplication, *35 enhanced activity <1% <1% 2-10% Gaston C and Kolesar J. Clin Adv Hematol Oncol. 2008;6:825-33. CYP2D6: Tramadol Experience Study population Design Treatment regimen Results: PM vs. EM Adult post-operative analgesia patients (N= 271) Prospective study of CYP2D6 PM and EM phenotypes 24-hour post-op tramadol dosing relative to phenotype Non-responders: 47% vs. 22% Needing rescue meds: 43% vs. 22% PM=Poor metabolizer EM=Extensive metabolizer Stamer UM, et al. Pain. 2003; 105: 231-8. PGx and Toxicity: Paclitaxel-Induced Peripheral Neuropathy (PIPN) • Expression of drug target – β-tubulin promoter gene, TUBB2A – SNP 112A > G, rs909965: ↑ gene transcription and protection from PIPN • Inadequate stimulus response – DNA repair gene, FANCD2 – ↑ expression led to ~80% ↑ in risk of Grade 3/4 neurological toxicities SNP=single nucleotide polymorphism Hertz D and McLeod H. J Hum Genet. 2013. 58(6): 346-52. CYP2C8*3 Genotype and PIPN Study population Design Treatment regimen Results European patients treated with paclitaxel for breast cancer (N=209); African American subset analysis (N=107) Genomic data collected for all newly diagnosed patients, 2005-2011 Neoadjuvant and adjuvant paclitaxelcontaining regimens •Carriers of *3 variant allele had ↑ risk of Grade 2+ neuropathy (HR 1.93; p=0.006) •Each *3 allele copy doubled a patient’s risk of Grade 2+ neuropathy (p=0.004) Hertz D, et al. Annals of Oncology. 2013. 24:1472-8. Grade 2+ Neuropathy in Mixed-Race Cohort (N=411) Hertz D, et al. Annals of Oncology. 2013. 24:1472-8. Opioid Absorption/Elimination: ABCB1 variations • Encodes P-glycoprotein (P-gp) 170 – Pumps drugs out of intracellular domain – Regulates CNS drug exposure • Fentanyl, methadone, and morphine are all P-gp substrates • Homozygous (TT) carriers of 3435C > T variant experience greater pain relief • 50-60% Caucasian population prevalence Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. ABCB1: Methadone Experience Adult methadone maintenance Study population patients (N= 120) Retrospective study of ABCB1 genetic variability Daily methadone dose relative to CC, CT, and TT carriers Wild-type CC: 98.3 mg ± 10.4 CT: 58.6 mg ± 20.9 Homozygous TT: 55.4 mg ± 26.1 Design Treatment regimen Results (p=0.029) . Coller JK, et al. Clin Pharmacol Ther. 2006; 80(6): 682-92. Opioid Receptor: OPRM1 variations • Encodes μ-opioid receptor • 118A > G variation = substitution of asparagine for aspartate • ↓ morphine, alfentanil, fentanyl, and methadone response • 20-30% population prevalence – 1-2% African Americans – 50% Japanese Argoff C. Clin J Pain. 2010;26(1):S16-20. Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. ABCB1/OPRM1: Morphine Pain Relief Study population Design Responder Groups Results Adult patients receiving morphine for acute pain control (N= 145) Prospective study of pain relief relative to ABCB1/OPRM1 genotype “Poor” pain control: CC/GG “Best” pain control: TT/AA Polymorphic association (p<0.00001) Sensitivity: ~100% Specificity: >70% Campa D, et al. Clin Pharm Ther. 2007; 83(4): 559-66. Catechol-O-methyltransferase (COMT) • Catecholamines are metabolized by COMT and involved in pain modulation • COMT activity may contribute to variable analgesic response • 1947G > A = 3-4 fold ↓ in COMT activity • Homozygous GG patients require higher morphine doses to achieve pain control Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. Morphine Dose Adjustments: Non-FDA Approved ABCB1 (TT) OPRM1 (GG) COMT (AA) Phenotype Cumulative Dosing Factor --- 2 --- Effective efflux pump 2 --- --- 0.67 ↓ COMT activity 0.67 0.67 --- --- Functional μ receptor 0.67 --- 2 0.67 Effective efflux/ ↓ COMT 1.33 0.67 2 --- Functional μ receptor/ effective efflux 1.33 0.67 --- 0.67 Functional μ receptor/ ↓ COMT 0.4 0.67 Functional μ receptor/ Effective efflux pump/ ↓ COMT 0.89 0.67 2 Lotsch J, et al. Pain. 2006; 121: 1-5. PGx Targets Summary Gene Variant Opioids Affected Outcome(s) CYP2D6 Poor; ultra rapid metabolizers Codeine, Tramadol, Oxycodone ↓ analgesia; excessive side effects ABCB1 3435C > T Morphine TT carriers experience greater pain relief OPRM1 118A > G Morphine, Alfentanil, Fentanyl, Methadone ↑ dose requirements due to ↓ efficacy COMT 1947G > A Morphine GG carriers experience less pain relief Jannetto P and Bratanow N. Expert Opin Drug Metab Toxicol. 2011; 7(6): 745-52. Select Variables Influencing Analgesic Response Pain related: •Kind of pain •Origin of pain •History of pain control Severity of: •Trauma •Surgery •Tissue damage Pharmacokinetics/ Pharmacodynamics Analgesic response Environmental factors: •Culture •Education •Family •Occupation Pharmacogenomics Psychological factors: •Depression •Anxiety •Coping strategies •Diagnosis Adapted from Stamer U, et al. Pharmacogenomics. 2010;11(6):843-64. Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results 2013 Pain Management Approvals • Zohydro® (ER hydrocodone) • Zubsolv® (buprenorphine and naloxone) ER=extended release Available at http://rogermontgomery.com/wp-content/uploads/2012/05/biotech-Fig-5.png. Accessed on 12.28.13. Biomarkers in Drug Discovery and Development Discovery 10,000 targets $ Development $$ Lead identification Target validation Preclinical safety Animal models $1.3 billion Clinical $$$ Proof of target Proof of mechanism Biomarkers Human tissue bank Proteomics Pathonomics Proteonics Clinical Samples: Tissue, blood, urine 250 in preclinical 5 in clinical 1 approved From The Role of Biomarkers in Drug Discovery and Development. Available at www.criver.com. Select Potential Future Targets Transduction TRPV1,2,3,4; P2X3; P2Y; Bradykinin B1/2; PGE2; NGF Modulation ATP; BNF; GABA; P2X4; P2X7; CCL2; fractalkine Transmission Nav1.7, -1.8, -1.9, -1.3; Kv9.1 Adapted from Heinzmann S and McMahon S. Current Opinion in Supportive and Palliative Care. 2011;5:111-5. Protein Kinase (PK) Inhibitors • PK are enzymes that enable/inhibit protein function and interactions • Profound upregulation of PK activity in models of neuropathic & inflammatory pain • p38 activation – allodynia/hyperalgesia; ↑ spontaneous pain perception • JNK expression – release of proinflammatory cytokines JNK=c-Jun n-terminal kinase Heinzmann S and McMahon S. Current Opinion in Supportive and Palliative Care. 2011; 5:111-5. Cytokine Antagonists • Cytokines induce chronic pain seen in inflammatory conditions • Cause swelling, tissue damage, and neuronal hypersensitivity • Known cytokines which enhance pain: – MCP-1 (and its receptor CCR2) – Fractalkine (and its receptor CX3CR1) – Tumor necrosis factor-α Heinzmann S and McMahon S. Current Opinion in Supportive and Palliative Care. 2011; 5:111-5. Nerve Growth Factor (NGF) Inhibitors • NGF mediates peripheral pain stimuli via small diameter sensory neurons • Upregulated in inflamed tissues resulting in hyperalgesia • Current trials exploring: – Neutralizing antibodies against NGF – NGF receptor (TrkA) antagonists Heinzmann S and McMahon S. Current Opinion in Supportive and Palliative Care. 2011; 5:111-5. Selective Sodium (Na) Channel Blockers • Nav1.7 mutation results in congenital insensitivity to pain • Nav1.7 plays large role in transduction of painful stimuli into action potentials • Many Na channel blockers available, but only few selective for 1.7 channel Heinzmann S and McMahon S. Current Opinion in Supportive and Palliative Care. 2011; 5:111-5. Tetrodotoxin (TTX) Derivatives • TTX-CINP-201 – Non-peptide, non-opioid neurotoxin – Derived from the puffer fish • Selectively blocks voltage-gated Na channels (Nav1.3?) • Phase II trial; recruiting participants – Safety and efficacy in chemotherapyinduced peripheral neuropathy (CIPN) Available at www.clinicaltrials.gov. Accessed on 12.29.13. TTX Mechanism of Action Available at www.mdpi.com. Accessed on 12.29.13. Spicamycin Derivatives • KRN-5500 – Non-opioid inhibitor of acetylcholinesterase and fatty acid amide hydrolase • Phase IIa, double-blind, placebo controlled – Refractory neuropathic pain & cancer (N=19) – 0.6-2.2 mg/m2; single, escalating doses • 24% ↓ pain intensity vs. 0% (P=0.03) • Most common AE: GI symptoms (92%) Weinstein S, et al. Journal of Pain and Symptom Management. 2012; 43(4): 679-93. Targeted Gene Therapy – NP2 • HSV vector delivers enkephalin to sensory nerves to block pain signals • Bypasses central nervous system • Avoids “typical” opioid adverse events • US Phase I study completed – No treatment-related AEs; no seroconversion • US Phase II study ongoing (N=32) – Randomized, double-blind, placebo controlled Available at www.clinicaltrials.gov. Accessed on 12.29.13. NP2 Mechanism of Action Available at www.paineurope.com. Accessed on 12.29.13. Investigational Analgesic Products Compound Molecular Target Comments Iosmapimod p38 kinase --- Tanezumab NGF Completed (osteoarthritis) Ralfinamide Nav1.7 Missed primary endpoint Lacosamide Nav1.7 --- TTX-CINP-201 Nav1.3? Phase II recruiting SSR 240612 Bradykinin-B1 receptor --- ADL5859/-747 δ-opioid receptor Failed in phase II trials KRN-5500 Acetylcholinesterase; fatty acid amide hydrolase CIPN phase II completed; FDA fast track designation NP2-Enkephalin Sensory nerves Phase II active; not recruiting Lotsch J and Geisslinger G. British Journal of Pharmacology. 2011: 163: 447-60. Available at www.clinicltrials.gov. Accessed on 12.29.13. Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results Objectives • Describe the principles and therapeutic implications of pharmacogenomics related to pain management • Review several novel analgesic drug targets identified from pharmacogenomic studies • Evaluate limitations and practical challenges of pharmacogenomic testing and adoption of results PGx – Healthcare Implications • • • • • • • Pain management treatment strategies Payer cost management strategies Medical informatics Patient prognostic expectations Drug research and development Accelerated drug approval Public health policy Benson A. Personalized Medicine in Oncology. 2012; 1(4): 1-5. Sadhasivam S and Chidambaran V. Pharmacogenomcis. 2012; 13(15): 1719-40. PGx Study Limitations • • • • • • Small sample sizes due to low prevalence Variability in polymorphisms assessed Multigenic factors altering drug responses Limited documentation of concurrent meds Different end points evaluated Population characteristics may affect study outcomes Walko C, et al. Journal of Pharmacy Practice. 2012; 25(4): 439-46. Practice-Based Evidence (PBE) • Prospective, observational cohort study • Data captured as part of routine clinical pain management • Complement randomized controlled trials • Help identify phenotypic and genotypic variables associated with favorable outcomes • Limited ability to infer causality • Hypothesis generation for subsequent validation via “traditional” methodology Bruehl S, et al. The Journal of Pain. 2013; 14(2): 103-13. PGx – Limitations to Implementation • • • • • • • Variable treatment response Effects of pain heterogeneity Limited ability to interpret test results ↑ costs/time associated with testing Healthcare providers’ knowledge limited Unknown drug resistance mechanisms New alleles of interest discovered daily Ma J, et al. Journal of Pharmacy Practice. 2012. 25(4):417-27. Personalized Analgesia Algorithm Considerations • Mandatory brain imaging – Dopaminergic modulation of opioid response • Endogenous opioid quantification – Reduced responsiveness to opioid analgesics • Effects of concurrent non-drug treatments – Acupuncture, relaxation training, exercise • Outcomes measurement – Functional magnetic resonance imaging (fMRI) Bruehl S, et al. The Journal of Pain. 2013; 14(2): 103-13. PGx Testing – Ethical Considerations Haga S and Burke W. Genet Med. 2008. 10(6): 391-5. Genomics Online Resources • www.cdc.gov/genomics/ • www.fda.gov/drugs/scienceresearch/resear chareas/pharmacogenetics/default.htm • ghr.nlm.nih.gov/glossary • www.genome.gov “If it were not for the great variability among individuals, medicine might as well be a science and not an art.” (Sir William Osler) 1892 Opioid Pharmacogenomics: The Path to Personalized Medicine Leigh M. Boehmer, Pharm.D., BCOP Clinical Pharmacist, Medical Oncology Barnes Jewish Hospital March 8, 2014