* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Chemistry 35 - Science-with
Survey
Document related concepts
Transcript
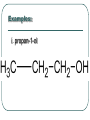
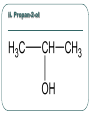
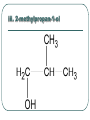
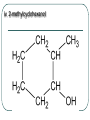
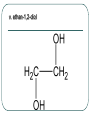
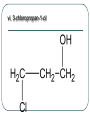
Chemistry 30 Organic Chemistry NOTES V. Hydrocarbon Derivatives 3. Alcohols a) Alcohols are compounds that have an OH (hydroxyl) group. Organic alcohols are non-ionic and do not produce OH- ions in aqueous solution. To name, number closest to the OH and give the ending –ol. Occasionally the OH is considered a branch called hydroxyl. Examples: i. propan-1-ol ii. Propan-2-ol iii. 2-methylpropan-1-ol iv. 2-methylcyclohexanol v. ethan-1,2-diol vi. 3-chloropropan-1-ol b) Alcohols may be subdivided into three classes based on the number of carbon atoms attached to the carbon with the hydroxyl group. c. Properties of Alcohols The presence of OH causes polarity and Hbonding. Therefore, the m.p. and b.p. are much higher, for example: methane b.p. = -16.2oC chloromethane b.p. = 23.7oC methanol b. p. = 65oC Alcohols are more soluble in water. However, as the hydrocarbon chains get longer, the polarity decreases so the solubility in water also decreases (i.e. methanol is completely soluble in water but octanol is barely soluble in water). There are 3 main types of reactions to consider: 1) Combustion 2) Elimination 3) Esterfication (later in notes) Elimination: alcohol becomes an alkene and water (water is eliminated) d. Uses of Alcohols i. Methanol (wood alcohol) - used to be obtained from the distillation of wood. Now it is made from: CO(g) + 2H2(g) CH3OH(l) It is used as a starting material for the production of acetic acid and many types of adhesives, fibers, and plastics. It is used in antifreeze and as duplicating fluid. It can also be used as a motor fuel. It is highly toxic to humans and can lead to blindness and death. ii. Ethanol (grain alcohol) - is the essential ingredient for alcoholic beverages, and is used in industry in its denatured state. Beverage – produced by yeast fermentation (beer 5.5%, wine 13%) before the yeast is killed by the alcohol. This reaction: Industry: motor fuel (gasohol), solvent, production of acetic acid. This reaction: Remainder of the Class Work on your workbooklet pages 21, page 24 and page 25 questions 2.a-f Finish up any unfinished work!