* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Acetaldehyde2
Isotopic labeling wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Microbial metabolism wikipedia , lookup
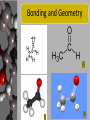
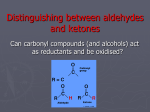
Acetaldehyde Regents Chemistry-Molecule Project 2009 Presented by Brett ApRoberts and Siggi Holmgren Molecule Statistics • • • • • Common Name Acetaldehyde Systematic Name Ethanal Molecular Formula C2H4O Gram Formula Mass 44.05 g/mol Elements in Molecule Carbon, Oxygen, and Hydrogen • Types of Bonds in Molecule Single and Double Covalent Bonds Bonding in Ethanal 1 Carbon can make four covalent bonds-Four unpaired electrons Hydrogen can make one covalent bonds-One unpaired electron Oxygen can make two covalent bonds-Two unpaired electrons Bonding and Geometry 2 3 4 Where can we find Ethanal? • Alcohol is converted into Ethanal in human metabolism. • Yeast and bacteria convert Ethanal to acetic acid in the vinegar making process. • Used in the production of wood and bookbinding glues known as polyvinyl acetates Relevance of Ethanal • Vinegar is a very common food in most cultures around the world. Importance to Human Survival • Ethyl alcohol, found in foods and beverages, must be broken down into Ethanal in our bodies or it would reach a toxic level that would be lethal. Metabolic Oxidation of Ethanal 5 This is the reaction pathway in our bodies and in vinegar production Life without Ethanal • I think food with be less interesting if Ethanal because we wouldn’t have vinegar for our salad dressing, pickles, or countless other recipes that include this sour product. • Ethanal is a colorless liquid that has a stong “green apple” smell and without it we might not have this flavor for our candy, gum, etc. When is Unity Benefitial? • When carbon, hydrogen, and oxygen bind together in Ethanal all of the atoms have full valence shells and benefit in being more stable than existing as atoms alone. • Since Ethanal reacts with oxygen to form acetic acid, the atoms can be more stable (benefit) when bonded together in acetic acid. Bibliography 1. The Alkane Series: Building Them From Scratch http://www.green-planet-solar-energy.com/electron-dot-diagram.html Accessed 12/06/09 2. Wikipedia Commons File:Acetaldehyde-2D.png http://commons.wikimedia.org/wiki/File:Acetaldehyde-2D.png Accessed 12/06/09 3. Background: Ethanol's Journey Through Your Body http://www.biochem.arizona.edu/classes/bioc462/462bh2008/462bhonorsprojects/462bhonors2 06/lowej/Acetaldehyde/ingestalcohol2.htm Accessed 12/06/09 4. The Race Equity Project http://lsnc.net/equity/category/environmental-justice/ Accessed 12/06/09 5. Science Aid-Alcohols http://scienceaid.co.uk/chemistry/organic/alcohols.html Accessed 12/31/09 6. International Agency for Research for Cancer http://monographs.iarc.fr/ENG/Monographs/vol71/mono71-11.pdf Accessed 12/31/09