* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Carbon and the Molecular Diversity of Life
Survey
Document related concepts
Transcript
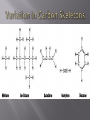
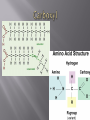
Honors Biology Unit 2: Biochemistry Monkemeier Organic compounds contain carbon and hydrogen and can exist as solids, liquids or gases. Scientists coined the classification “organic” because these molecules were synthesized in living systems. Compounds that are considered organic (only made by organisms) have been synthesized in the lab. Carbides, Carbonates, Oxides of Carbon (CO and CO2) and Cyanide are considered to be INORGANIC. Carbon has four valence electrons. To complete its valence shell, carbon forms four covalent bonds with other atoms. The tetravalence of carbon is at the center of carbon’s ability to form large and complex molecules with characteristic three-dimensional shapes and properties. Carbon atoms readily bond with each other, producing chains or rings of carbon atoms. These molecular backbones can vary in length, branching, placement of double bonds, and location of atoms of other elements. The simplest molecules The simplest organic molecules are hydrocarbons consisting of only carbon and hydrogen. The nonpolar C-H Bonds in hydrocarbon chains account for their hydrophobic behavior. When comparing the 4 categories of organic molecules (Carbohydrates, Lipids, Proteins and Nucleic Acids), Lipids contain the MOST C – H Bonds. WHAT do you know about lipids as a result of the high number of C-H bonds? Functional groups are groups of atoms covalently bonded that when attached to organic molecules provide them with characteristic properties. The functional groups studied in Chapter 4 are all HYDROPHILIC , therefore they INCREASE the solubility of the organic molecules in water. HYDROXYL: consists of an oxygen and hydrogen covalently bonded to the carbon skeleton. Organic compounds with hydroxyl groups are called ALCOHOLS and their names often end in –OL. Carbonyl Groups consist of carbon double-bonded to an oxygen. If the carbonyl group is at the end of the carbon skeleton, the compound is called an aldehyde. Otherwise, the compound is called a ketone. Carboxyl groups consist of a carbon double-bonded to an oxygen and also attached to a hydroxyl group. Compounds with a carboxyl group are called carboxylic acids or organic acids because they tend to dissociate and release H+. A phosphate group is bonded to the carbon skeleton its oxygen attached to the phosphorus atom that is bonded to three other oxygen atoms. The group is an anion. An amino group consists of a nitrogen atom bonded to two hydrogens. Compounds with an amino group, called amines, can act as bases. The nitrogen, with its pair of unshared electrons, can attract a hydrogen ion, becoming –NH3+ Sulfhydryl group consists of a sulfur atom bonded to a hydrogen atom. Thiols are compounds containing sulfhydryl groups. Isomers are compounds with the same molecular formula but different structural arrangements and, thus, different properties. Structural isomers differ in the arrangement of atoms and often in the location of double bonds. Enantiomers are left-handed and righthanded versions of each other and can differ greatly in their biological activity. An asymmetric carbon is one that is covalently bonded to four-different kinds of atoms or groups of atoms. Due to the tetrahedral shape of the asymmetric carbon, the four groups can be attached in spatial arrangements that are not super-imposable on each other. Molecules that are optical isomers, or mirror images, of one another. Enantiomers can be distinguished by the direction in which they rotate the plane of polarization of polarized light and are referred to, therefore, as being dextrorotatory (D-) or laevorotatory (L-). Enantiomers can exist when there is an asymmetric carbon atom within the molecule Geometric isomers have the same sequence of covalently bonded atoms but differ in spatial arrangement due to the inflexibility of double bonds. Carbon, Hydrogen, Oxygen, Nitrogen, and smaller quantities of sulfur and phosphorus, all capable of forming strong covalent bonds, are combined into the complex organic molecules of living matter. The versatility of carbon in forming four covalent bonds, linking readily with itself to produce chains and rings, and binding with other elements and functional groups makes possible the incredible diversity of organic molecules.