* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry
Survey
Document related concepts
Transcript
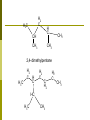
Organic Chemistry Chapter 25 Chemistry 100 Organic Chemistry Historically: the chemistry of products from plants and animals Today: The chemistry of carboncontaining compounds Wöhler (1828) made urea from ammonium cyanate Distinction between “natural” and “synthetic” chemicals disappeared. The world of plenty The American Chemical Society has a database with more than 11 million organic compounds Why so many? Because carbon can make chains and rings, and things Carbon Electronic configuration 1s22s22p2 Needs 4 electrons to complete the shell Electronegativity 2.5 so it forms covalent bonds with other elements 2s22p2 makes hybrid orbitals sp3 - tetrahedron Functional Groups We look at an organic molecule and ask “what functional groups does it have? Examples Alcohols have the OH group Amines have the NH2 group Unsaturated compounds have double or triple bonds C=C or CC Functional groups Acetic acid CH3COOH is a carboxylic acid - it has the -COOH group The compound CH3NH2 is methyl amine An amino acid like glycine (see picture) has both the carboxylate and the amino group Categorizing organic compounds There are so many we need a system to name them Unlike biologists we use English words, but sometimes you would not know this! Hydrocarbons contain only C and H Aliphatic (meaning derived from fats) Alkanes Alkenes Alkynes CnH2n+2 CnH2n CnH2n-2 Aromatic (yes - they smell) contain one or more benzene rings Alkanes CnH2n+2 n n n n n n n n n n = = = = = = = = = = 1 Methane (marsh gas) 2 Ethane 3 Propane 4 Butane 5 Pentane (liquid) 6 Hexane 7 Heptane 8 Octane 9 Nonane 10 Decane CH4 C 2 H6 C 3 H8 C4H10 C5H12 C6H14 C7H16 C8H18 C9H20 C10H22 Butane Condensed formula (two forms) C4H10 CH3CH2CH2CH3 CH3(CH2)2CH3 H H H H H C C C C H H H H H Lewis structural formula Iso-butane C4H10 CH3CH(CH3)CH3 We may think of this as CH3CH2CH3 with one of the H atoms substituted by a CH3 group CH3 CH CH3 CH3 H H H H C C C H H H C H H A branched chain H Alkyl groups CH3Cl methyl chloride CH3CH2CH2OH n-propyl alcohol Naming larger alkanes 1 2 3 4 Find the longest continuous chain. Use this as the base name Number the C atoms, starting from the nearest substitution Name and give location of each substitued group When more than one group at the same location: use di, tri, tetra, penta if they are the same name in alphabetical order, if different Naming Example H2 C H3C H C CH CH3 CH3 CH3 2,4-dimethylpentane H3C H2 C H C H2 C C H2 HC H3C CH3 H2 C CH3 Petroleum products Size Fraction Gas C1 to C5 Boiling-Point Range (0C) -160 to 30 Gasoline Kerosene, fuel-oil C5 to C12 C12 to C18 30 to 200 180 to 400 Lubricants Paraffins Asphalt C16 and up C20 and up C36 and up 350 and up Low-melting solids Gummy residues Uses Gaseous fuel, production of H2 Motor fuel Diesel fuel, furnace fuel, cracking Lubricants Candles, matches Surfacing roads Cycloalkanes CnH2n H2 C H2C CH2 H2C H2C C H2 H2 C CH2 H2 H2C C C CH2 H2 H2C H2C H2 C C H2 CH2 CH2 Cyclopropane cyclobutane cyclopentane cyclohexane Substituted cycloalkanes H2 C H2C C C CH3 H H2 H2 C H2C HC CH3 H2C CH CH3 ethylcyclopropane 1,2-dimethylcyclopentane Reactions of alkanes Combustion Substitution reactions C2H6 + Cl2 C2H5Cl + HCl Unsaturated hydrocarbons alkenes CnH2n double bond contain a H2C ethylene or ethene C2H4 propylene or propene C3H6 CH2 alkynes CnH2n-2 contain a triple bond acetylene C2H2 propyne C3H4 Sample alkenes CH 3 CH 3 2-methyl-2-butene H CH 3 H 3C CH 2 CH 3 2-pentene H CH 3CH=CH 2 H Geometrical isomers CH3CH=CHCH3 2-butene CH3 CH3 CH3 H H H H CH3 cis-2-butene trans-2-butene Addition reactions CH2=CH2 + Cl2 CH3CH=CHCH3 + H2 CH2Cl-CH2Cl CH3CH2CH2CH3 example of hydrogenation H3C H3C C C C C CH3 CH3 + + 2Cl2 Cl2 H3C H3C CCl CCl CCl2 CCl2 CH3 CH3 Aromatic hydrocarbons Functional Groups I Functional Groups II Alcohols; R-OH The hydroxyl or alcohol functional group -OH The OH group is polar; hydrogen bonding makes alcohols more soluble in water than in hydrocarbons CH3OH methyl alcohol, methanol, “wood alcohol”. Impurity in moonshine; causes blindness CH3CH2OH ethyl alcohol, ethanol, “alcohol” Prepared by fermentation of sugarcontaining plant material. Reactions with alcohols The OH group does NOT make alcohols basic. In fact, phenol is weakly acidic. An important reaction is esterification the formation of esters when an alcohol reacts with an acid. See notes on carboxylic acids Ethers R-O-R The -O- bridge CH3CH2-O-CH2CH3 is diethyl ether, or “ether”. Used in medicine but not as much as at one time. Very explosive! Ethers are used industrially as solvents Aldehydes and Ketones the carboxyl group C=O O R C H Aldehyde H C R Ketone C H formaldehyde O R O O H3C C H acetaldehyde O O H3C C C2H5 dimethyl ketone ethyl methyl ketone acetone H3C C CH3 Aldehydes Some aldehydes have common names like formaldehyde and acetyaldehyde. The other way to name them is with the al ending. CH3CH2CHO is propanal or propyl aldehyde Carboxylic Acids R-COOH O R C OH Carboxylic acid O H C OH methanoic acid formic acid O H3C C OH ethanoic acid acetic acid O CH3CH2 C OH propionic acid Alcohol Aldehyde Carboxylic acid OH OH R C H H C OH H H methanol H alcohol H3C C H H ethanol oxidation - remove hydrogen O O R C H Aldehyde H C O H formaldehyde H3C C H acetaldehyde oxidation - add oxygen R C O O O OH Carboxylic acid H C OH methanoic acid formic acid H3C C OH ethanoic acid acetic acid Esters Carboxylic acid + alcohol ester + water For organic chemists, reactions that generate water are called condensation reactions The condensation reaction is called esterification Fruity smell. Pentyl acetate (amyl acetate) gives bananas their smell but is very poisonous in large amounts. Esterification H O R C OH + HO C H O R' R C O C H carboxylic acid CH3CH2 C H ester alcohol OH + HO C H O H O CH3 CH3CH2 C O ethanol C H H propionic acid R' ester et hy lpropanat e CH3 Hydrolysis of ester Ester + water carboxylic acid + alcohol The reverse of esterification When done using a base (NaOH, for example), the reaction is called saponification. Soap was made by reacting fat (esters of stearic acid and glycerin) with NaOH to get sodium stearate. Amines R-NH2 , R2NH, R3N Organic chemistry’s base. Think of amines as substituted ammonia NH3 + R RNH2 R2NH R3N Just as NH3 is an proton acceptor making NH4+ , so amines are proton acceptors. They also react with carboxylic acids to give amides Horrid smell. One is called putricine, another cadaverine Amines CH3NH2 methylamine CH3CH2NH2 ethylamine CH3 (CH3)NH H3C dimethylamine N H Amides carboxylic acid + amine amide H O R C OH + H acetic acid R H C N OH + H N R' amide H O C R' amine carboxylic acid H3C N O CH3 methylamine H3C O H C N methylacetamide e s t er et hy l pr opanat e CH3 Amino acids A compound with the carboxylic acid group and the amine group H H2N C H O C Glycine OH Peptides &Proteins The amine group of one acid reacts with the carboxylic acid group of another to make a peptide When more molecules add on, we get a polypeptide Certain polypeptides are of biological importance - proteins Stereoisomers; chirality Carbohydrates Have the general formula Cn(H2O)n The simple sugars glucose and fructose are the basic building units of many carbohydrates. These sugar molecules can make rings. Disaccharides like table sugar (sucrose) consists of two rings joined together. When many rings join we get starch or cellulose. The difference is in how the rings are joined. We can digest starch but not cellulose