* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Part 3
Survey
Document related concepts
Transcript
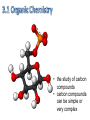
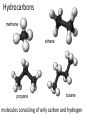
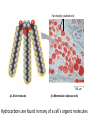
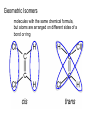
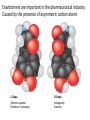
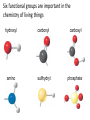
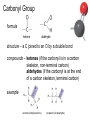
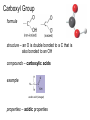
Topic 1:Chemicals of life 1. Molecules and Atoms 2. Water 3. Carbon and Other elements 3. Carbon and Other Elements 3.1 Organic Chemistry 3.2 Bonding with Carbon • • • Carbon Chains Hydrocarbons Isomers 3.3 Functional Groups • • • • • • Hydroxyl Carboxyl Carbonyl Amino Sulfhydryl Phosphate All living organisms are made up of chemicals based mostly on the element carbon, besides water • the study of carbon compounds • carbon compounds can be simple or very complex The Concept of Vitalism • organic compounds arise only within living organisms • disproven in 1953 when Miller synthesized the compounds in the laboratory C • carbon has four valence electrons • it can form four covalent bonds with a variety of atoms Carbon can make molecules of different length Name and Comments Molecular Formula Structural Formula Ball-and-Stick Model H (a) Methane CH4 H C H H (b) Ethane C2H6 H C C H H H (c) Ethene (ethylene) H H C2H4 H C H H C H Space-Filling Model Hydrogen Oxygen Nitrogen Carbon (valence = 1) (valence = 2) (valence = 3) (valence = 4) N C H O Carbon can bond covalently to many different elements Carbon can make Chains and Rings (a) Length H H H C C H H H H Ethane H H H C C C H H H H Propane H H (b) Branching H H H H H C C C C H H H H H H H H H H H H C C C C H H (d) Rings C C C H C C C H C C C H H H H 2-methylpropane (commonly called isobutane) H H H H H H C C C C H 1-Butene H H H H H H Butane (c) Double bonds C H H 2-Butene H H H H H Cyclohexane H C C C C H C Benzene • the skeletons of most organic molecules contain carbon • carbon skeletons vary in length, shape, and type of bonds Hydrocarbons methane ethane propane butane molecules consisting of only carbon and hydrogen Fat droplets (stained red) 100 µm (a) A fat molecule (b) Mammalian adipose cells Hydrocarbons are found in many of a cell’s organic molecules Isomers (a) Structural isomers H H H H H H H C C C C C H H H H H X C H H H C C C H H H C H C X H H CO2H CO2H C C H NH2 CH3 H X C C H (c) Enantiomers H X C (b) Geometric isomers H H H H H NH2 CH3 molecules with the same molecular formula but different structures and properties Structural Isomers molecules with the same chemical formula, but different arrangement of the atoms C4H10 They have different physical properties, ex. Melting point Geometric Isomers molecules with the same chemical formula, but atoms are arranged on different sides of a bond or ring Enantiomers are important in the pharmaceutical industry. Caused by the presence of asymmetric carbon atoms L-Dopa D-Dopa (effective against Parkinson’s disease) (biologically inactive) Estradiol OH CH3 HO the chemically reactive groups of atoms within an organic molecule that give it its chemical properties Female lion OH CH3 CH3 O Male lion Testosterone Six functional groups are important in the chemistry of living things hydroxyl carbonyl carboxyl amino sulfhydryl phosphate Hydoxyl Group formula structure – a H atom bonded to an O atom that is bonded to the carbon skeleton compounds – alcohols (names end in –ol) example H H H C C H H OH ethanol – in alcoholic beverages properties – polar, attracts H2O molecules, dissolves organic compounds Carbonyl Group formula structure – a C joined to an O by a double bond compounds – ketones (if the carbonyl is in a carbon skeleton, non-terminal carbon) aldehydes (if the carbonyl is at the end of a carbon skeleton, terminal carbon) H example H O C C H H C H H H H C C H H O C H H acetone (simple ketone) propanol (an aldehyde) Carboxyl Group formula structure – an O is double bonded to a C that is also bonded to an OH compounds – carboxylic acids example H H C H O C OH acetic acid (vinegar) properties – acidic properties Amino Group formula structure – a N atom bonded to two H atoms and to the carbon skeleton compounds – amines H O example C HO C H N H H glycine properties – acts as a base, can pick up a proton from solution Sulfhydryl Group formula structure – a S atom bonded to an atom of H compounds – thiols example H H H C C H H SH ethanethiol properties – can help stabilize protein structure by forming covalent bridges: disulfide bridges. Phosphate Group formula structure – a P atom is bonded to four O atoms, one O atom is bonded to the carbon skeleton compounds – organic phosphates OH OH H example H C C C H H H O O P O O glycerol phosphate properties – makes molecule into an anion, transfers energy between organic molecules