* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download 15_12_13rw
Survey
Document related concepts
Transcript
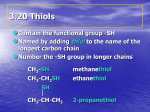
15.12 Thiols Copyright © The McGraw-Hill Companies, Inc. Permission required for reproduction or display. Nomenclature of Thiols 1) Analogous to alcohols, but suffix is -thiol rather than –ol. 2) Final -e of alkane name is retained, not dropped as with alcohols. CH3CHCH2CH2SH CH3 3-Methyl-1-butanethiol Properties of Thiols 1. Hydrogen bonding is much weaker in thiols than in alcohols (S—H bond is less polar than O—H). 2. Low molecular weight thiols have foul odors. 3. Thiols are stronger acids than alcohols. 4. Thiols are more easily oxidized than alcohols; oxidation takes place at sulfur. Thiols are Less Polar than Alcohols Methanol Methanethiol bp: 65°C bp: 6°C Thiols are Stronger Acids than Alcohols Have pKas of about 10; can be deprotonated in aqueous base. •• RS •• – •• H + •• OH stronger acid (pKa = 10) •• •• – RS •• •• + H •• OH •• weaker acid (pKa = 15.7) RS– and HS – are Weakly Basic and Good Nucleophiles C6H5S H H Cl C6H5SNa (75%) SN2 KSH Br SN2 SH (67%) Oxidation of Thiols Take Place at Sulfur •• RS •• thiol H •• RS •• •• SR •• disulfide Thiol-disulfide redox pair is important in biochemistry. Other oxidative processes place 1,2, or 3 oxygen atoms on sulfur. Oxidation of Thiols Take Place at Sulfur •• RS •• H thiol •• RS •• •• SR •• disulfide •• – •• O •• •• RS •• OH sulfenic acid + RS OH •• sulfinic acid •• – •• O •• 2+ RS OH •• O •• •• – sulfonic acid Example: Sulfide-disulfide Redox Pair SH O HSCH2CH2CH(CH2)4COH O2, FeCl3 S S O (CH2)4COH -Lipoic acid (78%) 15.13 Spectroscopic Analysis of Alcohols and Thiols Infrared Spectroscopy O—H stretching: 3200-3650 cm–1 (broad) C—O stretching: 1025-1200 cm–1 (broad) Figure 15.4 Infrared Spectrum of Cyclohexanol Francis A. Carey, Organic Chemistry, Fourth Edition. Copyright © 2000 The McGraw-Hill Companies, Inc. All rights reserved. Infrared Spectroscopy S—H stretching: 2550-2700 cm–1 (weak) 1H NMR Chemical shift of O—H proton is variable; depends on temperature and concentration. O—H proton can be identified by adding D2O; signal for O—H disappears (converted to O—D). H—C—O signal is less shielded than H—C—H. H 3.3-4 ppm C O H 0.5-5 ppm Figure 15.5 CH2CH2OH 10.0 9.0 8.0 7.0 6.0 5.0 4.0 3.0 Chemical shift (, ppm) 2.0 1.0 0 1H NMR Sulfur is less electronegative than oxygen, so is less deshielding. CH3CH2CH2CH2OH 3.6 CH3CH2CH2CH2SH 2.5 13C NMR Chemical shift of C—OH is 60-75 ppm. Deshielding effect of O is much larger than S. 14 19 35 62 CH3 — CH2 — CH2 — CH2 — OH CH3 — CH2 — CH2 — CH2 — SH 13 21 36 24 UV-VIS Unless there are other chromophores in the molecule, alcohols are transparent above about 200 nm; max for methanol, for example, is 177 nm. Mass Spectrometry of Alcohols Molecular ion peak is usually small. R A peak corresponding to loss of H2O from the molecular ion (M - 18) is usually present. Peak corresponding to loss of an alkyl group to give an oxygenstabilized carbocation is usually prominent. R R• CH2 •• OH •• •+ CH2 OH CH2 + OH •• ••