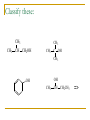* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Structure and Synthesis of Alcohols
Survey
Document related concepts
Transcript
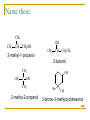
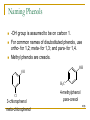
Structure and Synthesis of Alcohols Structure of Alcohols Hydroxyl (OH) functional group Oxygen is sp3 hybridized. => Classification Primary: carbon with –OH is bonded to one other carbon. Secondary: carbon with –OH is bonded to two other carbons. Tertiary: carbon with –OH is bonded to three other carbons. Aromatic (phenol): -OH is bonded to a benzene ring. => Classify these: CH3 CH3 CH3 CH CH2OH CH3 C OH CH3 OH OH CH3 CH CH2CH3 => IUPAC Nomenclature Find the longest carbon chain containing the carbon with the -OH group. Drop the -e from the alkane name, add -ol. Number the chain, starting from the end closest to the -OH group. Number and name all substituents. => Name these: CH3 CH3 CH CH2OH 2-methyl-1-propanol OH CH3 CH CH2CH3 2-butanol CH3 CH3 OH C OH CH3 2-methyl-2-propanol Br CH3 3-bromo-3-methylcyclohexanol => Unsaturated Alcohols Hydroxyl group takes precedence. Assign that carbon the lowest number. Use alkene or alkyne name. OH CH2 CHCH2CHCH3 4-penten-2-ol (old) pent-4-ene-2-ol (1997 revision of IUPAC rules) => Naming Priority Acids Esters Aldehydes Ketones Alcohols Amines Alkenes Alkynes Alkanes Ethers Halides => Hydroxy Substituent When -OH is part of a higher priority class of compound, it is named as hydroxy. Example: OH CH2CH2CH2COOH also known as GHB 4-hydroxybutanoic acid => Common Names Alcohol can be named as alkyl alcohol. Useful only for small alkyl groups. Examples: CH3 CH3 CH CH2OH isobutyl alcohol OH CH3 CH CH2CH3 sec-butyl alcohol => Naming Diols Two numbers are needed to locate the two -OH groups. Use -diol as suffix instead of -ol. OH HO 1,6-hexanediol => Glycols 1, 2 diols (vicinal diols) are called glycols. Common names for glycols use the name of the alkene from which they were made. CH2CH2 CH2CH2CH3 OH OH OH OH 1,2-ethanediol 1,2-propanediol ethylene glycol propylene glycol => Naming Phenols -OH group is assumed to be on carbon 1. For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4. Methyl phenols are cresols. OH OH H3C Cl 3-chlorophenol meta-chlorophenol 4-methylphenol para-cresol => Physical Properties Unusually high boiling points due to hydrogen bonding between molecules. Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. =>