* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Toxicology I
Metabolic network modelling wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Butyric acid wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biochemistry wikipedia , lookup
Citric acid cycle wikipedia , lookup
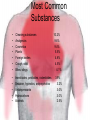
Poisoning/Overdose General Management Poisoning is Exposure to substance that is toxic in any amount Overdose Exposure to substance in excess amount resulting in toxic effects 1998 TESS* Data • • • • 2,241,082 reported human exposures 97.9 % at home Peak volume 4pm-10pm 91% of calls 8pm-midnight *The Toxic Exposure Surveillance System (US based) Exposures by Age • < 6 years old • < 3 years old Fatalities • • • • 775 fatalities 0.03% of total exposures ages 20 - 49 years = 56% >6 years = 2.1% 52.7% 39.6% Number of Substances • 92.8% of all cases--one substance • 44.7% of fatal cases-->2 substances Management Location • • • • Managed on site 75.2% Treated, released at ER only 12.3% Admitted to critical care 2.7% Refused referral * 2.0% Therapy • • • • • No therapy 11.9% Observation only 12.7% Decontamination only 59.6% Activated charcoal 6.8% Ipecac 1.2% Most Common Substances • • • • • • • Cleaning substances Analgesics Cosmetics Plants Foreign bodies Cough, cold Bites, stings • • • • • Insecticides, pesticides, rodenticides Sedative, hypnotics, antipsychotics Antidepressants Hydrocarbons Alcohols 10.2% 9.6% 9.4% 5.5% 4.6% 4.5% 4.1% 3.9% 3.2% 3.0% 3.0% 2.5% Largest Number of Deaths • • • • • • Analgesics Antidepressants Stimulants, street drugs Cardiovascular medication Sedatives, hypnotics Alcohols 264 152 118 118 89 56 Indicators • Sudden onset of CNS signs: – Seizures – Coma – Decreased LOC – Bizarre behavior • Sudden onset of: – Abdominal pain – Nausea – Vomiting Indicators cont • Sudden onset of unexplained illness • Bizarre, incomplete, evasive history • Trauma (>50% of adult trauma EtOH, drug-related) • Pediatric patient with arrhythmias History • • • • • What? How much? How long? Multiple substances? Treatment attempted? How? Whose advice? • Psychiatric history? • History of suicide? Scene Survey • Check out scene for : –1 –2 –3 –4 • Where do you look for clues? General Management • Support ABC’s – Secure airway, secure with advanced airway if needed – Ensure adequate oxygenation, ventilation – Maintain adequate circulation • Monitor ECG • Obtain vascular access • Manage hypotension initially with volume • Use vasopressors cautiously General Management • Keep patient calm • Maintain normal body temperature • Evaluate nature/toxicity of poison – Check container, package insert, poison center information – Treat the patient, not the poison General Management • Rule out (differential diagnosis) – Trauma – Neurological disease – Metabolic disease • Base general management on route of pois entry Poison Entry • Ingestion • Inhalation • Prevent absorption from GI tract • Remove from exposure; Support oxygenation, ventilation • Absorption • Remove from skin surface • Injection • Slow movement from injection site throughout body Ingested Poisons Objective Remove from GI tract before absorption occurs Ipecac • RARELY used anymore • If used, has to have been initiated within few minutes after ingestion • Vomiting in 20-30 minutes • Only removes about 32% of contaminate • Many contraindications Ipecac • Dose – 15 cc if 12 months to 12 years old – 30 cc if >12 years old • Follow with 2-3 glasses of water • Keep patient ambulatory if possible Ipecac • If no vomiting after 20 minutes, repeat • When emesis occurs, keep head down • Collect, save vomitus for analysis Ipecac • Contraindications – Comatose or no gag reflex – Seizing or has seized – Caustic (acid or alkali) ingestion – Low viscosity hydrocarbon ingestion – Late term pregnancy Ipecac • Contraindications – Severe hypertension, cardiovascular insufficiency, possible AMI – Ingestion of: • Strychnine • Phenothiazines (Thorazine, Stellazine, Compazine) • Tricyclic antidepressants • Iodides • Silver Nitrate Lavage • Commonly used in ED’s • Removes about 31% of substance • Helps get activated charcoal in patient, especially if patient is unconscious • Not helpful for sustained release tablets • Will not remove large tablets Activated Charcoal • Adsorbs compounds, prevents movement from GI tract • Very effective at adsorbing substances • Binds about 62% of toxin • Dose – 5 - 10X estimated weight of ingested chemical Activated Charcoal • Inactivates Ipecac • Do not give until vomiting stops • Do not give with – Cyanide – Methanol – Tylenol (+) • Containers must be kept airtight • Can be given PO via slurry or by NG Inhaled Poisons Objective: Move to fresh air; optimize ventilation and protect personnel from exposure Absorbed Poisons Objective: Remove poison from skin Liquid: Wash with copious amounts of water Powder: Brush off as much as possible, then wash with copious amounts of water Protect personnel from exposure Dilute / Irrigate / Wash • Use soap, shampoo for hydrocarbons • No need for chemical neutralization heat produced by reaction could be harmful Eye Irrigation • Wash for 15 minutes • Use only water or balanced salt solutions • Remove contact lenses • Wash from medial to lateral Examples of Specific Toxins Acids • Examples – Toilet bowl cleaner – Rust remover – Phenol (carbolic acid) – Hydrochloric acid • Severe burning of stomach • Absorption, systemic acidemia Acids • • • • • Loss of airway = most immediate threat Secure airway against edema IV with RL, NS for volume loss Emesis, gastric lavage contraindicated Dilution with water, milk NOT recommended Alkalis • Examples – Drain cleaner – Washing soda – Ammonia – Lye (sodium hydroxide) – Bleach (sodium hypochlorite) • Severe burning of esophagus, stricture formation Alkalis • • • • • Loss of airway = most immediate threat Secure airway against edema IV with LR, NS for volume loss Emesis, gastric lavage contraindicated Dilution with water, milk NOT recommended Hydrocarbons • Examples – Kerosene – Gasoline – Lighter fluid – Turpentine – Furniture polish Hydrocarbons • Signs/Symptoms – Choking, coughing, gagging – Vomiting, diarrhea, severe abdominal pain – Chemical pneumonitis, pulmonary edema If the patient is coughing, aspiration has occurred Methanol methyl alcohol wood alcohol wood naphtha Methanol • Sources – Industry – Household solvents – Paint remover – Fuel, gasoline additives – Canned heat – Windshield washer antifreeze Methanol • Toxic dose – Fatal oral: 30-240ml – Minimum: 100 mg/kg – Example • Windshield washer fluid 10% Methanol • 10 kg child needs only 10 cc to be toxic Methanol • Mechanism of toxicity – Methanol slowly metabolized to formaldehyde – Formaldheyde rapidly metabolized to formic acid • Acidosis • Ocular toxicity Methanol Metabolism H Methanol H C OH Alcohol dehydrogenase H O Formaldehyde H C H Aldehyde dehydrogenase O Formic Acid H C OH O H C _ O H+ Cyanide But first… • A little review of biochemistry and biophysics Staying alive requires energy... • The natural tendency of the universe is for things to become more disorderly. • This trend toward disorder is called entropy. • Complex systems (including us) don’t tend to last long, unless… • They have a constant supply of energy to combat entropy. Organisms capture and store the energy they need in the form of... Adenosine Triphosphate (ATP) • The “currency” cells use to pay off the energy debt built up fighting entropy. • Formed by capturing energy released as the cell breaks down large molecules through glycolysis and the Krebs Cycle. Putting It All Together • Cells have to have energy to stay alive. • Cells get energy by breaking down glucose in two phases: glycolysis and the Krebs Cycle. • Glycolysis yields 2 ATP and pyruvate. • Pyruvate is changed to acetate (acetyl-CoA) and sent to the Krebs Cycle. • The Krebs Cycle strips hydrogen and electrons off acetate and feeds them into the electron transport chain. • Movement of electrons down the transport chain releases energy which is trapped as ATP. • At the end of the chain, the electrons combine with hydrogen and oxygen to form water. • CN messes with this ! Cyanide • • • • • Chemical, plastic industries Metallurgy, jewelry making Blast furnace gases Fumigants, pesticides Present in various plants – apples, pears, apricots, peaches, bitter almonds • Remember there is CN gas released by BURNT plastics (e.g fire!!) Cyanide Antidote Kit • Amyl nitrite, sodium nitrite – Only be used in serious cyanide poisonings – Can induce life-threatening tissue hypoxia secondary to methemoglobinemia • Sodium thiosulfate – Can be used by itself – Is relatively benign Salicylates Salicylates • Examples – Aspirin – Oil of wintergreen • Uses – Analgesics – Antipyretics – Anti-inflammatories – Platelet function inhibitors Salicylates • Mechanism of Toxicity – Direct stimulation of respiratory center, causing respiratory alkalosis – Irritation of gastrointestinal tract, causing decreased motility, pylorospasm, nausea, vomiting, hemorrhagic gastritis – Decreased prothrombin levels/platelet dysfunction, causing prolonged clotting times – Uncoupling of oxidative phosphorylation Results of Oxidative Phosphorylation Uncoupling • ATP production decreases, resulting in CNS and cardiovascular failure. • Cells attempt to compensate by increasing the rate they process glucose anaerobically through glycolysis. • Lactic and pyruvic acids accumulate, leading to metabolic acidosis. • Hypoglycemia results as liver sugar stores are depleted. • In absence of sugar cells begin to metabolize lipids, ketone bodies are produced, acidosis worsens. • Energy normally trapped as ATP is wasted as heat, causing a rise in body temperature. • The rise in body temperature accelerates metabolism, increasing tissue oxygen demand and worsening acidosis. Salicylates Clinical Presentation: Acute Toxicity – – – – Vomiting Lethargy Hyperpnea Respiratory alkalosis – Metabolic acidosis – – – – – Coma Seizures Hypoglycemia Hyperthermia Pulmonary edema Salicylates • Clinical Presentation: Chronic Toxicity – Usually young children, confused elderly – Confusion, dehydration, metabolic acidosis – Higher morbidity, mortality than acute overdose – Cerebral, pulmonary edema more common Salicylates • Acute Toxicity Management – Oxygen, monitor, IV – GI tract decontamination – Activated charcoal – Replace fluid losses, but do NOT overload – Control hyperthermia Salicylates • Acute Toxicity Management – Bicarbonate for metabolic acidosis – D50W for hypoglycemia – Diazepam for seizures Acetaminophen Acetamophen • Examples – Tylenol – Tempra – Many drugs contain this also • Uses – Analgesic – Antipyretic Acetaminophen • Mechanism of toxicity – N-acetyl p-benzoquinonimine, normal product of acetaminophen metabolism, is hepatotoxic – Normally is detoxified by glutathione in liver – In overdose, toxic metabolite exceeds glutathione capacity, causes liver damage Acetaminophen • Management – Induce emesis – Do NOT give activated charcoal in general – Give specific acetaminophen antidote Acetaminophen • The specific antidote for acetaminophen toxicity. Mucomyst • N-acetylcysteine Another sulfur-containing amino acid Substitutes for glutathione. Allows continued detoxification of NAPBQI. 140mg/kg initially followed by 70mg/kg every 4 hours 17 times. Tastes, smells like rotten eggs Mix with chilled fruit juice to decrease odor, taste More to come • Review – Cocaine OD – TCA OD – Opoid OD – MDMA - E – GHB