* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Amines - Chemistry Geek
Homoaromaticity wikipedia , lookup
Hydroformylation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Aromaticity wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Aromatization wikipedia , lookup
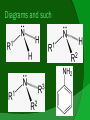
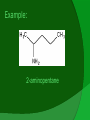
(not to be confused with Anime) Vs. What are they? Amines are functional groups that contain a basic nitrogen with a lone pair. They are derivatives of ammonia (NH3) with one or more of the hydrogens replaced with a substituent such as an alkyl group. Properties Usually basic Due to hydrogen bonding, attached amines raise the boiling point of compounds Attaching amine groups onto some gases turns them into liquids (i.e. diethylamines, triethylamines) Gaseous amines smell like ammonia; liquid amines smell like fish Soluble in water! Aromatic amines = toxic Types of Amines Primary – One hydrogen is replaced by a substituent group Secondary – Two hydrogens are replaced by substituent groups Tertiary – Three hydrogens are replaced by substituent groups Aromatic (Aniline) – NH2 attached to a benzene Diagrams and such Uses/Applications PROTEINS! Dyes Drugs – Many drugs are designed to mimic natural amine neurotransmitters. Gas treatment – Used to refine natural gas by removing CO2 and H2S from gas streams Fabric softener, protection against gamma radiation, etc! Drugs continued… Amphetamine and methamphetamine are controlled substances Chlorpheniramine is an antihistamine that helps to relieve allergic disorders Chlorpromazine is a tranquillizer that sedates without inducing sleep Ephedrine and Phenylephrine, as amine hydrochlorides, are used as decongestants Naming: IUPAC If you consider a primary amine to be an amino group attached to an alkane, you would simply name it as you would an alkane derivative Name the longest carbon chain the parent chain Count the amine group as an attached group with prefix amino Example: 2-aminopentane IUPAC continued: To name secondary and tertiary amines, use N to show the location of an alkyl group that is attached to the nitrogen atom. The N group prefix is placed before the amino prefix. Try it! N-ethyl-N-methyl-1-aminoethane Common naming: You can also name primary amines by naming the alkyl group, followed by the word "amine". This should be written all as one word, even though you may sometimes see the parts written separately. Your turn: methylisopropylbutylamine Priority Carboxylic acids, esters, aldehydes, ketones, alcohols all have higher priority Amines Ethers, Alkenes, Alkynes, Nitro, Alkanes all have lower priority