* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Ch 16 Amines - Tennessee Wesleyan College
Survey
Document related concepts
Transcript
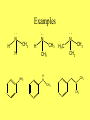
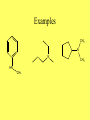
Ch 16 Amines Homework problems: 16.9, 16.10, 16.21, 16.25, 16.39, 16.40 Amines • Carbon, Hydrogen, and Oxygen are the 3 most common elements in organic compounds • Because of the wide distribution of amines in the biological world, Nitrogen is the fourth most common element. Structure and Classification • The functional group for amines is a Nitrogen bonded to alkyl groups and/or Hydrogen N R R= C or H R R • Amines are classified as 1o, 2o, or 3o based on The number of alkyl groups bonded to the Nitrogen!! N H H CH3 N H CH3 N CH3 H3 C CH3 CH3 Further Classification • Amines are further classified as Aliphatic or Aromatic • Aliphatic Amines: all carbons bonded to the nitrogen are derived from alkyl groups • Aromatic Amines: one or more of the carbons bonded to the nitrogen are in an aromatic ring Examples N H CH3 H N N CH3 H CH3 NH2 H N CH3 H3 C CH3 CH3 CH3 N CH3 Heterocyclic Amines • An amine in which the Nitrogen is a member of a ring is classified as a Heterocyclic Amine • When the Nitrogen is part of a regular ring, it is a Heterocyclic Aliphatic Amine • When the Nitrogen is part of an aromatic rind, it is a Heterocyclic Aromatic Amine Examples N N H N H Pyrrolidine Piperidine Heterocyclic Aliphatic Amines N Pyridine N N N Pyrimidine** N N H Purine** Heterocyclic Aromatic Amines * Important building blocks for the amino bases in DNA and RNA DRUGS!!!! • Amines are typically very important in drugs, both legal and illegal • See Chemical Connections 16A and 16B to learn more!!! IUPAC Nomenclature • IUPAC names for 1o aliphatic amines are derived just like alcohols, you drop the -e from the parent name and add -amine • Use numbers to indicate location of amino NH2 NH2 NH2 H2 N Aromatic Amines • IUPAC keeps the common name Aniline: NO 2 CH3 NH2 NH2 NH2 o 2 and o 3 Amines • 2o and 3o amines are named as Nsubstituted primary amines • The largest alkyl group bonded to the Nitrogen is taken as the parent amine • The smaller groups are named as substituents and their locations are indicated with an N- Examples CH3 N N HN CH3 CH3 Amine Salts • When four atoms or groups are bonded to the Nitrogen, the Nitrogen bears a positive charge. H H H H N H H N CH3 H • The cation is usually associated with an anion and presented as a salt Naming Amine Salts • The ending -amine is replaced by -ammonium and the name of the anion is added after. H N H Cl N H Br Physical Properties • Like Ammonia, low molecular weight amines have very sharp odors • Amines are polar because of the difference in electronegativity between Nitrogen and Hydrogen and also between Nitrogen and Carbon. • 1o and 2o amines have a Hydrogen bonded to a Nitrogen so they are capable of Hydrogen bonding to each other • 3o Amines do not have a Hydrogen bonded to Nitrogen so they can not Hydrogen bond to one another. • All classes of amines can H-Bond with water and are therefore soluble in water!! Reactions of Amines • Basicity – Due to the lone pair of electrons on the Nitrogen, amines are weak bases and aqueous solutions of amines are basic. – Recall, a base is a substance that will accept a hydrogen in a reaction. H N H H + H OH N H H OH Reactions with Acids • Amines react completely with strong acids to form water soluable salts. HO H HO HO NH2 + H-Cl HO Norepinephrine (only slightly soluable in water) H HO H2O NH3 Cl HO Norepinephrine hydrochloride (completely soluable in water)