* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture14
Survey
Document related concepts
Transcript
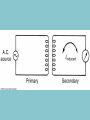
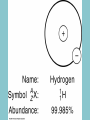
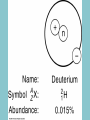
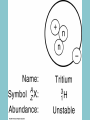
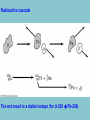
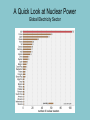
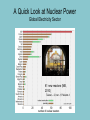
Electricity is transmitted over high voltage lines A Transformer: Converts a current at one voltage into a current in a separate circuit at a different voltage Entirely depends on the relative numbers of coils A Transformer: Converts a current at one voltage into a current in a separate circuit at a different voltage Entirely depends on the relative numbers of coils Remember….power is proportional to the square of the current: P = I2R The power lines have a small resistance, and so the power lost to heat will be P = I2R Need to reduce the current to reduce power lost! For the Transformer, V(in) x I(in) = V(out) x I(out) If you go from 120 V to 120,000 V, your current is 1000 times less, and your power loss is a million times less!! Demand for Electricity changes over the period of a day Taum Sauk Reservoir : New Construction Taum Sauk Reservoir Failure: Dec. 14, 2005 Taum Sauk Reservoir Failure: Dec. 14, 2005 Taum Sauk Reservoir Failure: Dec. 14, 2005 Taum Sauk Reservoir Failure: Dec. 14, 2005 New Taum Sauk Reservoir “Standard Model” of particles in the universe Structure of Neutron and Proton Structure of Helium Structure of Helium Why isn’t the atomic mass 4 (actually 4.0028 amu)? Structure of Helium Why isn’t the atomic mass 4 (actually 4.0028 amu)? Nuclear Binding Energy! Why isn’t the atomic mass 4 (actually 4.0028 amu)? Nuclear Binding Energy! Mass of one neutron: 1.00866 amu Mass of one proton: 1.00728 amu 2 x neutron + 2 x proton = 4.0319 amu Why isn’t the atomic mass 4 (actually 4.0028 amu)? Nuclear Binding Energy! Mass of one neutron: 1.00866 amu Mass of one proton: 1.00728 amu 2 x neutron + 2 x proton = 4.0319 amu SO….Nuclear Binding Energy = 4.0319 – 4.0028 = 0.029 amu Or, 4.82 x 10-29 kg But, E = mc2 = (4.82 x 10-29 kg)(3 x 108 m/s)2 = 4.3 x 10-12 J (Joining the neutrons and protons to make helium nucleus releases the energy) Why isn’t the atomic mass 4 (actually 4.0028 amu)? Nuclear Binding Energy! Mass of one neutron: 1.00866 amu Mass of one proton: 1.00728 amu 2 x neutron + 2 x proton = 4.0319 amu SO….Nuclear Binding Energy = 4.0319 – 4.0028 = 0.029 amu Or, 4.82 x 10-29 kg But, E = mc2 = (4.82 x 10-29 kg)(3 x 108 m/s)2 = 4.3 x 10-12 J One gram of helium (by fusion) = burning 23 tons of coal Radioactive Decay: Alpha, Beta, Gamma Radioactive Decay: Alpha Decay (atom loses helium nucleus) ( = Helium nucleus) Radioactive Decay: Beta Decay (atom loses electron; neutron turns to proton) ( = Electron) Radioactive Decay: Electron Capture (proton turns into neutron) Zircon Crystals: Good for trapping in Uranium and Lead atoms (The oldest known zircon crystal in the solar system, from an Apollo 17 Moon rock: 4.42 billion years old) Meteorites – oldest rocks on Earth Allende meteorite (carbonaceous chondrite) Radioactive cascade The end result is a stable isotope (for U-238 Pb-206) A Quick Look at Nuclear Power U.S. Electricity Sector - Nuclear • nuclear power is major player in U.S. electricity industry – 19. 4 % of electricity • third major source behind: – coal: 46.6 % – natural gas: 21.5 % • characteristics: – despite no new plants since 1970s, percentage of electricity it produces has been growing – many plants being re-licensed for another 20-30 years – U.S. safety record has been stellar • no fatalities, no injuries A Quick Look at Nuclear Power U.S. Electricity Sector A Quick Look at Nuclear Power Global Electricity Sector A Quick Look at Nuclear Power Global Electricity Sector A Quick Look at Nuclear Power Global Electricity Sector A Quick Look at Nuclear Power Global Electricity Sector 61 new reactors (NEI, 2010) Taiwan – 2; Iran -1; Pakistan -1 Nuclear Physics Fundamental Forces Nuclear Physics Balancing Nuclear Forces Nuclear Physics Binding Curve Nuclear Physics Nuclear Transformations • the nuclear structure of atoms is changed by three different mechanisms: – fission: splitting of heavy nuclei into two lighter ones with the releases of neutrons and energy • spontaneous • neutron-induced – fusion: combining of two nuclei to make a new, heavier nuclei • new nuclei has less mass than sum of two original nuclei – radioactive decay: spontaneous emission of either particle or electromagnetic radiation by nuclei • particle: alpha, beta, electron capture • electromagnetic: gamma • these processes are not influenced by physical conditions, e.g. pressure, temperature, etc. Nuclear Physics Binding Curve Fission Fusion Nuclear Physics Nuclide Chart Nuclear Physics Nuclide Chart