* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development 2015
Plant tolerance to herbivory wikipedia , lookup
History of herbalism wikipedia , lookup
Evolutionary history of plants wikipedia , lookup
Plant stress measurement wikipedia , lookup
Plant nutrition wikipedia , lookup
Venus flytrap wikipedia , lookup
History of botany wikipedia , lookup
Flowering plant wikipedia , lookup
Ornamental bulbous plant wikipedia , lookup
Plant defense against herbivory wikipedia , lookup
Plant secondary metabolism wikipedia , lookup
Plant evolutionary developmental biology wikipedia , lookup
Plant use of endophytic fungi in defense wikipedia , lookup
Plant physiology wikipedia , lookup
Plant reproduction wikipedia , lookup
Plant breeding wikipedia , lookup
Plant ecology wikipedia , lookup
Sustainable landscaping wikipedia , lookup
Plant morphology wikipedia , lookup
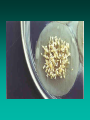
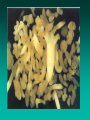
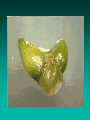
Somatic Embryogenesis l Parthenocarpy l Apomixis l In vitro somatic embryogenesis Soybean – Wayne Parrot, UGA Somatic Embryos • Bipolar • Not connected to explant or callus cells by vascular tissue • In most woody plants, tissue must be juvenile or reproductive Indirect Somatic Embryogenesis Induction • Auxins required for induction –Proembryogenic masses form –2,4-D most used –NAA, dicamba also used Development • Auxin must be removed for embryo development • Continued use of auxin inhibits embryogenesis • Stages are similar to those of zygotic embryogenesis – Globular – Heart – Torpedo – Cotyledonary – Germination (conversion) Maturation • Require complete maturation with apical meristem, radical, and cotyledons • Often obtain repetitive embryony • Storage protein production necessary • Often require ABA for complete maturation • ABA often required for normal embryo morphology – Fasciation – Precocious germination Germination • May only obtain 3-5% germination • Sucrose (10%), mannitol (4%) may be required • Drying (desiccation) – ABA levels decrease – Woody plants – Final moisture content 10-40% • Chilling – Decreases ABA levels – Woody plants Rubber tree from somatic embryo CIRAD Factors that Influence SE • Genotype • Growth regulators • Carbon source • Nitrogen Maturation and Germination (Conversion) Micropropagation “… the art and science of multiplying plants in vitro.” Rapid clonal in vitro propagation of plants: •from cells, tissues or organs •cultured aseptically on defined media •contained in culture vessels •maintained under controlled conditions of light and temperature Toward Commercial Micropropagation 1950s Morel & Martin 1952 Meristem-tip culture for disease elimination Morel 1960 Wimber 1963 Disease eradication & in vitro production of orchids Commercialization of Micropropagation 1970s & 1980s Murashige 1974 Broad commercial application Clone Genetically identical assemblage of individuals propagated entirely by vegetative means from a single plant. Conventional Propagation • Cuttings • Budding, grafting • Layering Conventional Propagation Advantages • Equipment costs minimal • Little experience or technical expertise needed • Inexpensive • Specialized techniques for growth control (e.g. grafting onto dwarfing rootstocks) Micropropagation Advantages • From one to many propagules rapidly • Multiplication in controlled lab conditions • Continuous propagation year round • Potential for disease-free propagules • Inexpensive per plant once established Micropropagation Advantages • Precise crop production scheduling • Reduce stock plant space • Long-term germplasm storage • Production of difficult-to-propagate species Micropropagation Disadvantages • Specialized equipment/facilities required • More technical expertise required • Protocols not optimized for all species • Plants produced may not fit industry standards • Relatively expensive to set up? Micropropagation Applications • Rapid increase of stock of new varieties • Elimination of diseases • Cloning of plant types not easily propagated by conventional methods (few offshoots/ sprouts/ seeds; date palms, ferns, nandinas) • Propagules have enhanced growth features (multibranched character; Ficus, Syngonium) Explant •Cell, tissue or organ of a plant that is used to start in vitro cultures •Many different explants can be used for micropropagation, but axillary buds and meristems are most commonly used Choice of explant Desirable properties of an explant: • Easily sterilizable • Juvenile • Responsive to culture • Importance of stock plants • Shoot tips • Axillary buds • Seeds • Hypocotyl (from germinated seed) • Leaves Methods of micropropagation • Axillary branching >95% of all micropropagation Genetically stable Simple and straightforward • Adventitious shoot formation Efficient but prone to genetic instability • Somatic embryogenesis Little used, but potentially phenomenally efficient Axillary shoot proliferation Growth of axillary buds stimulated by cytokinin treatment; shoots arise mostly from pre-existing meristems Shoot Culture Method Overview •Clonal in vitro propagation by repeated enhanced formation of axillary shoots from shoot-tips or lateral meristems cultured on media supplemented with plant growth regulators, usually cytokinins. •Shoots produced are either rooted first in vitro or rooted and acclimatized ex vitro ADVANTAGES •Reliable rates and consistency of shoot multiplication •3 -8 fold multiplication rate per month •Pre-existing meristems are least susceptible to genetic changes • mericloning A propagation method using shoot tips in culture to proliferate multiple buds, which can then be separated, rooted and planted out • First commercially used with orchids conventional propagation rate of 1 per year. • Through protocorms, 1,000,000 per year. Corm (Swollen stem) Chop into pieces Maturation Axillary shoot production • • • • • • Selection of plant material Establish aseptic culture Multiplication Shoot elongation Root induction / formation Acclimatization Selection of plant material • Part of plant • Genotype • Physiological condition • Season • Position on plant • Size of explant Physiological state - of stock plant • Vegetative / Floral • Juvenile / Mature • Dormant / Active • Carbohydrates • Nutrients • Hormones Stage 1 Disinfestation • Stock plant preparation • Washing in water • Disinfecting solution • Internal contaminants • Screening Mother Block: A slowly multiplying indexed and stabilized set of cultures Serve as source of cultures (explants) for Stage II multiplication Stage I - Sterilization • Bacteria and fungi will overgrow the explant on the medium unless they are removed • Pre-treatments to clean up the explant • Detergents • Sterilants and Antibiotics Pre-treatments • Transfer plants to a greenhouse to reduce endemic contaminants • Force outgrowth of axillary buds • Washing removes endemic surface contaminants • Antibiotics, fungicides, Admire, others STAGE II: Shoot Production • Stage II selection of cytokinin type and concentration determined by: •Shoot multiplication rate •Length of shoot produced •Frequency of genetic variability •Cytokinin effects on rooting and survival Problems • Vitrification – a glassy appearance to tissue • Long acclimation time • Callus formation leading to mutations STAGE II: Shoot Production •Subculture shoot clusters at 4 -5 week intervals •3 -8 fold increase in shoot numbers • Number of subcultures possible is species/cultivar dependent STAGE II: Shoot Production STAGE III: Pretransplant (rooting) Goals: •Preparation of Stage II shoots/shoot clusters for transfer to soil (prehardening) •Elongation of shoots prior to ex vitro rooting •Fulfilling dormancy requirements Shoot elongation ... • Basal ‘hormone free’ medium • Gibberellins • Carry-over of hormones Root initiation • Auxins • Charcoal • C : N ratio • Light / darkness • Initiation vs growth • Juvenility / rejuvenation • Genotype STAGE III: Pretransplant (rooting) STAGE IV: Transfer to Natural Environment Ultimate success of shoot culture depends on ability to acclimatize vigorously growing quality plants from in vitro to ex vitro conditions Stage IV STAGE IV: Transfer to Natural Environment Acclimatization: •Process whereby plants physiologically and anatomically adjust from in vitro to ex vitro cultural and environmental conditions •Two reasons micropropagated plants may be difficult to acclimatize ex vitro: Low photosynthetic competence (heterotrophic nutrition) Poor control of water loss