* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Characteristic
Survey
Document related concepts
Transcript
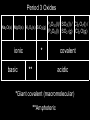
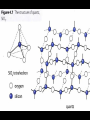
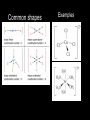
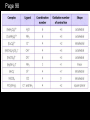
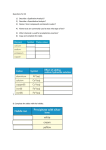
IB Assessment 13.1.1 Explain the physical states (under standard conditions) and electrical conductivity (in the molten state) of the chlorides and oxides of the elements in period 3 in terms of their bonding and structure. IB Assessment 13.1.2 Describe the reactions of chlorine and the chlorides referred to in 13.1.1 with water. Period 3 Oxides P4O10(l)/ SO3(l) / Cl2 O7(l) / Na2O(s) MgO(s) Al2O3(s) SiO2(g) P4O6(l) SO2 (g) Cl2 O(g) ionic basic * ** covalent acidic *Giant covalent (macromolecular) **Amphoteric Period 3 Oxides P4O10(l) SO3(l) / MgO(s Na2O(s) Al2O3(s) SiO2(g) ) /P4O6(l) SO2 (g) Melting Point High (ionic bonding) Electrical Conductivity (molten state) Good Reacts Yes with water (basic soln.) No Cl2O7(l) / Cl2O(g) Low High (intermolecular forces) Poor None No Yes (acidic solutions) Period 3 Chlorides MgCl2(s Al2Cl6(s) NaCl(s) SiCl4(l) ) (AlCl3) Structure Melting Point Electrical Conductivity (molten state) Reacts with water ionic high (801 oC - 714 oC good S2Cl2(l) (dissolves easily) Cl2(g) covalent Moderat e (178 oC) poor low (-70 oC to -112 oC) none slowly no weakl Nature of neutra y solution PCl3(l)/ PCl5(s) HCl produced acidic (both oxidized & reduced) IB Assessment 13.2.1 List the characteristic properties of transition elements. Transition element: Any element that contains partially filled d orbitals in their atoms or ions. (Allows electrons to “transition” from one d orbital to another.) Characteristic properties of transition elements: • variable oxidation numbers (charges) • form complex ions • form colored compounds • have catalytic properties IB Assessment 13.2.2 Explain why Sc and Zn are not considered to be transition elements. Both Sc and Zn do not contain ions with partially filled d orbitals: IB Assessment 13.2.3 Explain the existence of variable oxidation number in ions of transition elements. Transition elements can be oxidized to lose 1, 2, 3, or more electrons. IB Assessment 13.2.4 Define the term ligand. Ligand: A molecule or negative ion that donates a pair of electrons* to a central metal ion to form a dative covalent (coordinate) bond. *a Lewis base IB Assessment 13.2.5 Describe and explain the formation of complexes of d-block elements. p97 Complex ion: A central ion, usually a transition metal ion, surrounded by a fixed number of ligands which form dative covalent (coordinate) bonds with the d orbitals. Common shapes Examples Page 98 IB Assessment 13.2.6 Explain why some complexes of dblock elements are colored. p100 The color of transition metals is due to a split in the d sub-level, each with slightly different energies.... 3+(aq) Example Ti(H2O) 6 . ...the electronhere: transitions between light istoabsorbed and its themGreen (from lower higher energy) complimentary colorof (purple) results in an absorbance specificis colorsemitted. of light. TiCl3(aq) IB Assessment 13.2.7 State examples of the catalytic action of transition elements and their compounds. You only need to know those examples shown in the video... add them to your notes as we come to them! IB Assessment 13.2.8 Outline the economic significance of catalysts in the Contact and Haber processes. Process Haber Contact The catalytic properties of many transition elements and their compounds help to make many important industrial processes more efficient and economical: Catalyst Product Uses Fe V2O5 ammonia production of fertilizers, plastics, drugs, explosives... sulfuric acid production of detergents, dyes, explosives, drugs, other chemicals... ; the electrolyte in the lead-acid storage (car) batteries Process Haber Contact The catalytic properties of many transition elements and their compounds help to make many important industrial processes more efficient and economical: Catalysts Product Uses Fe V2O5 ammonia production of fertilizers, plastics, drugs, explosives... sulfuric acid The most produced chemical in the world... It’s production is a strong indicator of the strength of a country’s economy.