* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 1 Structure and Bonding
Bond valence method wikipedia , lookup
Persistent carbene wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metalloprotein wikipedia , lookup
Hydroformylation wikipedia , lookup
Metal carbonyl wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
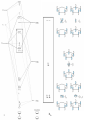
Chapter 13 Lecture 2 More Ligand Types I. Ligands with Extended p Systems A. Linear p Systems 1) Ethylene (C2H4) a) Single p-bond composed of two overlapping p-orbitals b) One bonding and one antibonding p molecular orbital 2) Allyl Radical (C3H5) CH CH2 HC HC CH CH2 3) 1,3-Butadiene 4) Other extended p systems H2 C CH2 CH2 HC HC HC CH CH HC HC CH2 CH CH HC CH2 CH2 B. Cyclic p Systems 1) Cyclopropene a) Similar construction of orbitals as in linear systems b) Degenerate orbitals have the same number of nodes 2) Polygon Method for finding cyclic p system MO’s a) Draw molecule as a polygon with vertex down b) One MO per vertex gives energy ordering and degeneracy c) Number of nodes increases as energy increases C. Bonding Between Metals and Linear p Systems 1) Ethylene Complexes a) Sidebound geometry is most common b) Bonding: s-donation from p MO, p-acceptance from p* MO c) Coordination weakens C=C bond (137.5 pm, 1516 cm-1) compared to free ethylene (133.7 pm, 1623 cm-1) 2) p-Allyl Complexes a) Can be trihapto: both s- and p-bonding b) Can be monohapto: s-bonding only from sp2 hybrid orbital (120o bond angle) c) The lowest energy MO provides s-bonding, highest energy MO = p-acceptor p3 is a p-acceptor p2 can be donating or accepting depending on metal e- distribution p1 is a s-donor 3) Other linear p-system coordination D. Bonding in Cyclic p Systems 1) Cyclopentadienyl = Cp = C5H5- is the most important cyclic ligand 2) Ferrocene Synthesis: FeCl2 + 2 NaC5H5 a) Called metallocene or sandwich complex b) 18-electron complex: Fe2+ = d6 and 2 Cp x 6 ec) Bonding Group Orbitals of 2 eclipsed Cp rings D5h 0-Node Group Orbitals (h5-C5H5)2Fe + 2 NaCl d) Matching with metal d-orbitals: dyz orbital example e) MO Description i. 6 strongly bonding MO’s hold electrons from Cp ligands ii. 8 antibonding orbitals are empty iii. 5 mid-range energy orbitals holding metal d-electrons Reactivity i. Follows 18-electron rule, but not inert ii. Ligand reactions on Cp ring are most common reactions f) D5h II. M—C Single, Double, and Triple Bonds A. Metal Alkyl Complexes 1) Grignard Reagents: X—M—CH2CH2CH2CH3 2) Bonding in Transition Metal Complexes a) s-donation from C sp3 hybrid orbital b) 2 electron, -1 charge for electron counting 3) Synthesis a) ZrCl4 + 4 PhCH2MgCl Zr(CH2Ph)4 b) Na[Mn(CO)5] + CH3I CH3Mn(CO)5 + NaI 4) Other M—C single bond ligands B. Metal Carbene Complexes 1) M=C counted as 2 electron, neutral ligand in electron counting 2) Schrock Alkylidenes: only H or C attached to the carbene Carbon 3) Fisher Carbenes: heteroatom attached to the carbene Carbon (our focus) a) s-bond from C sp2 hybrid to metal b) p-bond from C p-orbital(s) c) Heteroatom delocalizes p-system to 3 atoms, stabilizing it by resonance C. Metal Carbyne Complexes 1) First synthesis in 1973 by Lewis Acid attack on carbene complex 2) Bonding a) 180o bond angle and short bond length confirm triple bond b) 3 electron, 0 charge for electron counting III. Spectroscopy of Organometallic Complexes A. Infrared Spectroscopy 1) Number of Bands is determined by group theory (chapter 4 procedure) a) Monocarbonyl = 1 band only b) Dicarbonyl i. Linear arrangement = 1 band only ii. Bent arrangement = 2 bands c) 3 or more Carbonyls: table 13.7 in your book 2) Position of IR Bands a) Electron Density determines Wavenumbers Cr(CO)6 n = 2000 cm-1 [V(CO)6]- n = 1858 cm-1 [Mn(CO)6]+ n = 2095 cm-1 b) c) Bonding Mode Other ligands B. NMR Spectroscopy 1) Proton NMR a) Hydride Complexes M—H hydrogen strongly shielded (-5 to –20 ppm) b) M—CH3 hydrogens 1-4 ppm c) Cyclic p system hydrogens 4-7 ppm and large integral because all the same 2) 13C NMR a) Useful because “sees” all C ligands (CO) and has wide range (ppm) b) CO: terminal = 195-225 ppm, bridging slightly larger C. HC 3 S Examples N C 1) [(Cp)Mo(CO)3]2 + tds Product? H3C S tds a) Data: 1H NMR: 2 singlets at 5.48 (5H) and 3.18 (6H) IR: 1950, 1860 cm-1 Mass = 339 b) Solution: proton nmr 5.48 = Cp, 3.18 = ½ tds IR: at least 2 CO’s Mass: 339 - (Mo=98) – (Cp=65) – 2(CO) = 120 = ½ tds Product = (Cp)Mo(CO)2(S2CN(CH3)2) S CH3 C N S CH3 CO OC 2) OC Re CO Br C O PPh3, toluene, II + III O I: proton = 4.83 (4H), carbon = 224, 187, 185, 184, 73 II: proton = 7.62-7.41 m (15H), 4.19 (4H) carbon: 231, 194, 189, 188, 129-134,72 III: proton = 7.70-7.32 m (15H), 3.39 s (2 H) carbon: 237, 201,193,127-134, 69 Solution: 224 = M=C; 184-202 = CO; 73 = CH2CH2 CO CO OC Ph3P Re CO II OC Br C O O Ph3P Re Br C PPh3 O III O