* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 2Cu 2+ + O 2 2
Survey
Document related concepts
Transcript
Amino acids/subunit
153
113
628
Sipuncula
Priapulida
marine worms
Brachiopoda
Annelida: Magelona papillicornis
Iron porphyrin
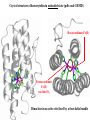
Active site
Monomeric
Dinuclear copper
Multimeric
N. Terwilliger, J. Exp. Biol.201, 1085–1098 (1998)
Dinuclear
iron
http://notes.chem.usyd.edu.au/course/codd/CHEM3105/Metalloproteins3.pdf
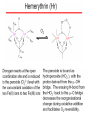
Crystal structure of hemerytrhin in unloaded state (pdb-code 1HMD)
Hexacoordinate Fe(II)
Pentacoordinate
Fe(II)
can bind O2
Dinuclear iron active site fixed by a four-helix bundle
http://notes.chem.usyd.edu.au/course/codd/CHEM3105/Metalloproteins3.pdf
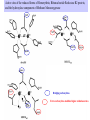
Active sites of the reduced forms of Hemerythrin, Ribonucleotide Reductase R2 protein,
and the hydroxylase component of Methane Monooxygenase
Bridging carboxylates
Extra carboxylates stabilize higher oxidation states
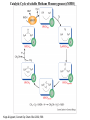
Catalytic Cycle of soluble Methane Monooxygenase (sMMO)
Kopp & Lippard, Current Op. Chem. Biol. 2002, 568
Remember:
Hr and sMMO share the main features:
a four-helix-bundle surrounding a Fe-(carboxylato)2-Fe core
but differ in the particular environment of the Fe centers:
-Hr coordination sphere is more histidine rich
-Hr permits only terminal O2-coordination to a single iron, while sMMO
diiron center presents open or labile coordination sites on both Fe
-sMMO shows much greater coordinative flexibility upon oxidation
-The larger number of anionic ligands allows sMMO to achieve the FeIV
oxidation state needed for oxidation methane.
Intermezzo: Bioligands
Histidin
pKa (His+) = 6.0 neutral at pH 7, but can be easily protonated,
can serve as „proton shuttle“
Both tautomers are found as ligands
pKa (His) = 14.4 rarely exists in deprotonated form as
bridging ligand (in Cu-Zn superoxide-dismutase)
Aspartate & Glutamate
pKa (COOH) = 3.9
pKa (COOH) = 4.1
at pH 7 anionic even without coordination to a metal atom
Cysteinate
Cys
pKa (SH) = 8.3
neutral at pH 7. Coordination to a metal atom stabilizes
anionic form.
Tyrosinate
Tyr
pKa (TyrH) = 10.1
neutral at pH 7. Coordination to a metal atom stabilizes
anionic form.
Can be oxidized to a radical Tyr· (see RNR-R2)!
Intermezzo: Bioligands
Methionine
neutral, „soft“ ligand
prefers FeII to FeIII
occurs in cytochromes (electron transfer proteins) where it stabilizes the
lower oxidation state
General rules governing the Redox-potential in a transition-metal complex
Larger number of ligands
Anionic ligands
Soft ligands (methionine)
stabilize higher oxidation states
stabilize the lower oxidation state
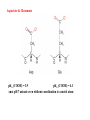
Porphyrins
vinyl
farnesyl
(isoprenoid chain)
methyl
formyl
Heme a
Amino acids/subunit
153
113
628
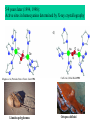
Panulirus interruptus
Linulus polyphemus
Octopus dofleini
Megathura crenulata
Chemistry enabling O2 transport by hemocyanin
Loading O2:
2Cu+ + O2 2Cu2+ + O22Red.
Ox.
Ox.
Red.
Unoading O2:
2Cu2+ + O22- 2Cu+ + O2
Ox.
Red.
Red.
Ox.
strong oxidants
Vybrané standardní redukční potenciály při 25°C:
F2 (g) + 2 e– = 2 F– (aq)
+ 2.87
MnO4 – + 8H+ + 5e– = Mn 2+ + 4H2O
+ 1.51
Cl2 (g) + 2 e– = 2 Cl– (aq)
+ 1.36
Pt2+ (aq) + 2 e– = Pt (s)
+ 1.18
Br2 (g) + 2 e– = 2 Br– (aq)
+ 1.07
Fe3+ (aq) + e– = Fe2+ (aq)
+ 0.77
I2 (g) + 2 e– = 2 I– (aq)
+ 0.54
2 H2O + O2 (g) + 4 e– = 4 OH– (aq)
+ 0.41
stronger oxidant O2 + 2H+ + 2e- = H2O2
+ 0.35
Cu2+ (aq) + 2 e– = Cu+ (aq) stronger oxidant + 0.15
2 H+(aq) + 2 e– = H2 (g)
0.00
Fe2+ (aq) + 2 e– = Fe (s)
- 0.45
Zn2+ (aq) + 2 e– = Zn (s)
- 0.76
Al3+ (aq) + 3 e– = Al (s)
- 1.67
Mg2+ (aq) + 2 e– = Mg (s)
- 2.37
Na+ (aq) + e– = Na (s)
- 2.71
Li+ (aq) + e–
=
Li (s)
- 3.04
strong reductants
(at pH 7)
Chemistry enabling O2 transport by hemocyanin
O2 stronger oxidant
Loading O2:
Cu+ stronger reductant
OK
2Cu+ + O2 2Cu2+ + O22Red.
Ox.
Ox.
Red.
Unloading O2:
2Cu2+ + O22- 2Cu+ + O2
Ox.
Red.
Red.
Ox.
would procede in reverse direction
in aqueous solutions at pH 7
But: Tetrahedral Cu- environment in hemocyanin favors Cu+ !
The potential of the Cu 2+/Cu+ couple shifts to 0.3-0.4 V
The potentials of both half-reactions become similar
The whole reaction becomes reversible
General rules governing the Redox-potential in a transition-metal complex
Larger number of ligands
Anionic ligands
Soft ligands (methionine)
Coordination geometry
imposed by the protein
stabilize higher oxidation states
stabilize the lower oxidation state
can stabilize the higher or the lower oxidation state
Hemocyanin: History
1878
Leon Federicq: Sur l‘hemocyanine, substance nouvelle
de sang de Poulpe (Octopus vulgaris)
(Compt. Rend. Acad. Sci. 87, 996-998)
Discovery
1901
M. Henze: Zur Kenntniss des Haemocyanins
Z. Physiol. Chem. 33, 370
Hemocyanin contains copper
1940
W. A. Rawlinson, Australian J. Exp. Biol. Med. Sci. 18,
131
Oxy-hemocyanin is diamagnetic
http://webdoc.sub.gwdg.de/diss/2003/ackermann/ackermann.pdf
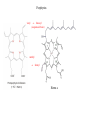
On the search for functional hemocyanin model compounds
Karlin et al., JACS 1988, 110, 3690’3692
The first model complex showing reversible O2 binding by a dicopper unit
However, this complex differs from oxy-Hc:
Cu-Cu[Å]
1
1
υ(O-O)[cm-1]
4.36
Oxy-Hc 3.5-3.7
834
744-752
Karlin et al., J. Am. Chem. Soc. 1988, 110, 3690-3692
UV-VIS
440(2000)
525(11500)
590(7600)
1035(160)
340(20000)
580(100)
Model complex showing reversible O2 binding and similar features to Hc
Kitajima et al., J. Am. Chem. Soc.
1989, 111, 8975-8976
Cu-Cu[Å]
2
3.56
υ(O-O)[cm-1]
741
UV-VIS
349(21000)
551(790)
2
Oxy-Hc
3.5-3.7
744-752
340(20000)
580(100)
Functional hemocyanin models
[(tmpa)2Cu2O2]2+
[Cu{HB(3,5-iPr2pz)3}]2(O2)
Karlin et al., JACS 1988, 110, 3690’3692
Kitajima et al., JACS 1989, 111, 8975-8976
UV-Vis absorption spectra of the oxy forms of hemocyanin and tyrosinase
ps→d
pv→d
d→d
5-9 years later (1994, 1998):
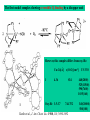
Active sites in hemocyanins determined by X-ray crystallography
Magnus et al.,Proteins Struct. Funct. Gen.1994
Limulus polyphemus
Cuff et al.,J.Mol.Biol.1998
Octopus dofleini
http://pollux.chem.umn.edu/~kinsinge/new_homepage/research/gss_presentation_3/sld019.htm
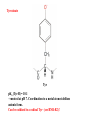
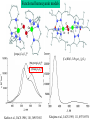
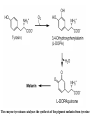
L-DOPAquinone
The enzyme tyrosinase catalyzes the synthesis of the pigment melanin from tyrosine
Tyrosinase versus Hemocyanin
The coupled binuclear copper sites in tyrosinase and hemocyanin are very similar.
Why is then tyrosinase capable of reacting with substrates while hemocyanin is not?
Solomon (Angew. Chem. Int. Ed. Engl. 2001, 40, 4570-450):
Difference in accessibility of the active site
Hypothesis, 1980:
Solomon et al., JACS 1980, 102, 7339-7344, p.7343
Angew. Chem. Int. Ed. 2001, 40, 4570-4590
Proof, 1998 (J. Biol. Chem. 273, 25889-25892):
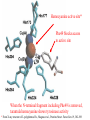
Hemocyanine active site*
Phe49 blocks access
to active site
When the N-terminal fragment including Phe49 is removed,
tarantula hemocyanine shows tyrosinase activity
* From X-ray structure of L.polyphemus Hc., Magnus et al., Proteins Struct. Funct.Gen.19, 302-309
An earlier model for hemocyanin...
…turned out to be a model for the enzyme tyrosinase!
Karlin et al., JACS 1984, 106, 2121-2128
Conclusions
In many cases, metalloproteins use the same or similar active site
for different purposes.
The strategies to confer a particular activity to a given site include
- Allowing/disallowing access of substrates to the active site
(including the dynamics of diffusion of substrate/product)
-Modifying the electrostatic potential by mutating the amino acids
coordinated to the metal or surrounding the binding pocket
-Architecture of the binding pocket defines substrate selectivity
and affects energy of transition states→governs reaction outcome