* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download sfc113 96-97
Survey
Document related concepts
Transcript
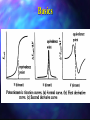
Titrimetric Analysis Quantitative chemical analysis carried out determining the volume of a solution accurately known concentration which required to react quantitatively with measured volume of the substance to determined. by of is a be Classification Neutralisation Reactions Complex Formation Reactions Redox Reactions Precipitation Reactions Basics Equivalence and end points Standards Basics Equivalence and end points Precise and accurate titrations require the reproducible determination of the end point which either corresponds to the stoichiometric point of the reaction or bears a fixed and measurable relation to it. Basics Equivalence and end points Monitor a property of the titrand which is removed at the end point. Monitor a property which is readily observed when excess titrant has been added. Two main methods Coloured indicators Electrochemical techniques. Basics Colour Change Indicators Common to a wide variety of titrations. In general terms a visual indicator is a compound that changes from one colour to another as its chemical form changes. = InB + nX where X may be H+, Mn+ or e-, and the colour is sensitive to the presence of H+, Mn+, oxidants or reductants. InA Basics An indicator constant is defined as: KIn = [InB][X]n / [InA] [X]n = KIn ([InB] / [InA]) npX = pKIn + log10([InB] / [InA]) pH = pKa + log10 ([InB] / [InA]) Basics Potentiometric Measurements Measuring the change in potential during the titration. Acid-base titrations. Precipitation Redox titrations. titrations. Basics Monitor the change of Ecell during the course of a titration where the indicator electrode responds to one of the reactants or the products. A plot of Ecell against the volume of titrant is obtained. Precision of better than 0.2%. Basics Basics The Nernst Equation aA + bB + …+ ne- = xX + yY + ... x[Y]y... [X] RT 0 E=E ln a[B]b... [A] nF Basics RT/F ln 10 = 0.059158 V thus: E = E0 - (0.059 V/n) log10 And [X]x[Y]y... [A]a[B]b... E = E0 at unity concentrations Basics Conductimetric Indication The electrical conductance of a solution is a measure of its current carrying capacity and is determined by its total ionic strength. It is a non-specific property. Conductance is defined as the reciprocal of resistance (Siemans, -1). Basics A conductance cell consists of two platinum electrodes of large surface area. 5-10 V at 50 -10,000 Hz is applied. Control of temperature is essential. Basics Acid-base titrations especially at trace levels. Relative precision better than 1% at all levels. Rate of change of conductance as a function of added titrant used to determine the equivalence point. High concentrations of other electrolytes can interfere. Basics Basics Standards Certain chemicals which are used in defined concentrations as reference materials. Primary standards. Secondary standards. Basics Primary Standards Available in pure form, stable and easily dried to a constant known composition. Stable High in air. molecular weight. Readily soluble. Undergoes stoichiometric and rapid reactions. Basics Acid-base Na2CO3, reactions. Na2B4O7, KH(C8H4O4), HCl (cbpt.) Complex formation reactions. AgNO3, NaCl Precipitation reactions. AgNO3, KCl Redox reactions. K2Cr2O7, Na2C2O4, I2 Basics Secondary Standards A substance that can be used for standardisations, and whose concentration of active substance has been determined by comparison to a primary standard. Classification Neutralisation Reactions Complex Formation Reactions Redox Reactions Precipitation Reactions Neutralisation Titrations The neutralisation reactions between acids and bases used in chemical analysis. These reactions involve the combination of hydrogen and hydroxide ions to form water. Neutralisation Titrations For any actual titration the correct end point will be characterised by a definite value of the hydrogen ion concentration. This value will depend upon the nature of the acid and the base, the concentration of the solution and the nature of the indicator. Neutralisation Titrations A large number of substances called neutralisation indicators change colour according to the hydrogen ion concentration of the solution. The end point can also be determined electrochemically by either potentiometric or conductimetric methods. Theory of Indicator Behaviour An acid/base indicator is a weak organic acid or a weak organic base whose undissociated form differs in colour from its conjugate base or conjugate acid form. The behaviour of an acid type indicator is described by the equilibrium; Theory of Indicator Behaviour In- + H3O+ HIn + H2O The behaviour of an base type indicator is described by the equilibrium; In + H2O InH+ + OH- Theory of Indicator Behaviour The equilibrium constant takes the form: [H3O+][In-] = Ka [HIn] Rearranging: -] [HIn [H3O+] = Ka [In-] Theory of Indicator Behaviour pH (acid colour) = -log(Ka . 10) = pKa +1 pH (base colour) = -log(Ka / 10) = pKa -1 Therefore; indicator range = pKa ± 1 Theory of Indicator Behaviour The human eye is not very sensitive to colour change in a solution containing In- and HIn. Especially when the ratio [In-] / [HIn] is greater than 10 or less than 0.1. Hence the colour change is only rapid within the limited concentration ratio of 10 to 0.1. Theory of Indicator Behaviour Neutralisation Titrations Strong acids and bases Weak acids Weak bases Polyfunctional acids Applications Neutralisation Titrations Strong acids and bases. When both reagent and analyte are strong electrolytes, the neutralisation reaction can be described by the equation: H3O+ + OH- 2H2O Neutralisation Titrations The H3O+ concentration in aqueous solution comprises of two components. The reaction of the solute with water. The dissociation of water. [H3O+] = CHCl + [OH-] = CHCl [OH-] = CNaOH + [H3O+] = CNaOH Neutralisation Titrations Using these assumptions you can calculate the pH of a titration solution directly from stoichiometric calculations and therefore simulate the titration curves. This is useful in determining the correct indicator for a new titration. Neutralisation Titrations Neutralisation Titrations Examples: HCl, HNO3 NaOH, KOH, Na2CO3 Standards:anhydrous boiling HCl. Na2CO3 and constant Neutralisation Titrations Weak acids and bases Examples Ethanoic acid Sodium cyanide Four types of calculation are required to derive a titration curve for a weak acid or base. Neutralisation Titrations Solution contains only weak acid. pH is calculated from the concentration and the dissociation constant. After additions of the titrant the solution behaves as a buffer. The pH of each buffer can be calculated from there analytical concentrations. At the equivilence point only salt is present and the pH is calculated from the concentration of this product. Beyond the equivilence point the pH is governed largely by the concentration of the excess titrant. Neutralisation Titrations Effect of Concentration Effect of reaction completeness Indicator choice; Feasibility of titration Neutralisation Titrations Neutralisation Titrations Polyfunctional acids and bases Typified by more than one dissociation reaction. Neutralisation Titrations Phosphoric acid Yield multiple end points in a titration. Neutralisation Titrations Neutralisation Titrations Sulphuric Acid Unusual because one proton behaves as a strong acid and the other as a weak acid (K2 = 1.20 x 10-2). Neutralisation Titrations Applications: Determination of the concentration of analytes which are either acid or bases. Determination of analytes which can be converted to acids or bases. Complexometric Titrations Titrations between cations and complex forming reagents. The most useful of these complexing agents are organic compounds with several electron donor groups that can form multiple covalent bonds with metal ions. Complexometric Titrations Most metal ions react with electron-pair donors to form coordination compounds or complex ions. The donor species, or LIGAND, must have at least one pair of unshared electrons available. Complexometric Titrations Inorganic Ligands Water Ammonia Halides Organic Ligands Cyanide Acetate Complexometric Titrations The number of bonds a cation forms with an electron donor is called the COORDINATION NUMBER. Typical values are 2, 4 and 6. The species formed as a result of coordination can be electrically positive, neutral or negative. Complexometric Titrations Complexometric methods have been around for more than a century. Rapid expansion in the 1940’s based on a class of coordination compounds called CHELATES. Complexometric Titrations A chelate is produced when a metal ion coordinates to two or more donor groups within a single ligand. For example the copper complex of glycine. Complexometric Titrations NH2 Cu2+ + 2 H C C OH H O C O O O C Cu C N H2 H2 O + 2H + N C H2 H2 Complexometric Titrations A ligand with a single donor group is called unidentate. Glycine is bidentate. Tri, tetra, penta and hexadentate chelating agents are also known. Complexometric Titrations Multidentate ligands have two advantages over unidentate ligands. They react more completely with cations to provide a sharper endpoint. The reaction is a single step process. Complexometric Titrations Tertiary amines that also contain carboxylic acid groups form remarkably stable chelates with many metal ions. Ethylenediaminetetraacetic Acid EDTA HOOCCH2 CH 2CO O H N HOOCCH2 CH2 CH2 N CH 2CO O H Complexometric Titrations EDTA can complex a large number of metal ions. Approximately 40 cations can be determined by direct titration. EDTA is usually used as the disodium salt, Na2H2EDTA H2EDTA2- + M2+ [M(EDTA)]2- + 2H+ Complexometric Titrations Because EDTA complexes most cations, the reagent might appear at first glance to be totally lacking in selectivity. However, great control can be acheived by pH regulation and the selection of suitable indicators. Complexometric Titrations Indicators are generally complexing agents which undergo a colour change when bonded to a metal ion. H2EDTA2- + [M(Ind)] [M(EDTA)]2- + Ind2- + 2H+ Complexometric Titrations Typical indicators are: Murexide Solochrome black Calmagite Bromopyrogallol Xylenol orange red Complexometric Titrations Typical applications: Determination of cations Hardness of water Redox Titrations Basics Potassium Permanganate Potassium Dichromate Cerium IV Iodine Redox Titrations Basics Electrode Indicators Potentials Redox Titrations Electrode Potentials Derived from Nernst equation. Calculations of cell potentials leads to theoretical titration curves. EOX = ERED = Esystem Redox Titrations Redox Titrations Indicators Potentiometric Coloured EIn indicators = EOX = ERED = Esystem Specific: Starch Oxidation / Reduction Indicators Redox Titrations Redox Titrations Potassium Permanganate MnO4- + 8H+ + 5e- Mn2+ + 4H2O Standardisation Sodium oxalate or arsenic (III) oxide Many Analyses Redox Titrations Hydrogen Peroxide: 2MnO4- + 5H2O2 + 6H+ 2Mn2+ + 5O2 + 8H2O Nitrites: 2MnO4- + 5NO2- + 6H+ 2Mn2+ + 5NO3- + 3H2O Redox Titrations Persulphates: Add an excess of iron (II) S2O82- + 2Fe2+ + 2H+ 2Fe3+ + 2HSO4 The excess iron (II) is determined by back titration against standardised permangenate. MnO4- + 8H+ + 5Fe2+ Mn2+ + 5Fe2+ + 4H2O Redox Titrations Potassium Dichromate CrO72- + 14H+ + 6e- 2Cr3+ + 7H2O Standardisation Against metallic iron 1 mole K2CrO7 = 6 moles Fe Redox Titrations Iron (II): CrO72- + 14H+ + 6Fe2+ 2Cr3+ + 6Fe3+ 7H2O Indicators include N-phenylanthranilic acid and sodium diphenylamine sulphonate. Chlorates: Reduced The with an excess of iron (II) excess iron (II) is determined by back titration against standardised dichromate. Redox Titrations Iodine Iodometric Titrations I2 + 2e- 2I- Standardisation Standardised sodium thiosulphate or arsenic (III) oxide Many Analyses Redox Titrations Hydrogen Peroxide: Thiosulphates: H2O2 + 2I- + 2H+ I2 + 2H2O 2S2O32- + I2 S4O62- + 2I- Hydroxyl Groups: 2OH- + I2 IO- + H2O + 2I- Redox Titrations Others: Copper Dissolved oxygen Chlorine Arsenic (V) Sulphides etc.......... Redox Titrations Cerium (IV) Sulphate Very strong oxidising agent (1.43V) Ce4+ + e- Ce3+ Standardisation Sodium oxalate or arsenic (III) oxide Many Analyses Precipitation Titrations Titrations between analytes and reagents resulting in the formation of a precipitate. The most useful of these precipitating reagents is silver nitrate. Titrimetric methods based upon the use of silver nitrate are sometimes called Argentometric titrations. Precipitation Titrations Used for the determination of many anions including: halides divalent anions mercaptans certain fatty acids Precipitation Titrations Precipitation titrations are based on the SOLUBILITY PRODUCT of the salt, KSP. The smaller KSP, the less soluble the silver salt and the easier it is to determine the endpoint Precipitation Titrations Endpoint determination is by coloured indicators (usually back titrations) or turbidity methods. The most accurate is the VOLHARD METHOD. Precipitation Titrations VOLHARD METHOD A back titration of thiocyanate ions against the excess silver ions using an iron (II) salt as the indicator. Precipitation Titrations Ag+ + SCN- AgSCN Fe3+ + SCN- FeSCN2+ Blood Red