* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Jahn–Teller effect wikipedia , lookup
Hydroformylation wikipedia , lookup
Cluster chemistry wikipedia , lookup
Metal carbonyl wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Spin crossover wikipedia , lookup
Metalloprotein wikipedia , lookup
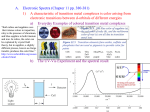
Coordination Chemistry Transition elements: partly filed d or f shells Which elements should be considered as transition elements? Why do we consider the 1st row separately from others? The d block: • The d block consists of three horizontal series in periods 4, 5 & 6 – 10 elements in each series – Chemistry is “different” from other elements – Special electronic configurations important • Differences within a group in the d block are less sharp than in s & p block • Similarities across a period are greater What is a transition metal? • Transition metals [TM’s] have characteristic properties – e.g. coloured compounds, variable oxidation states • These are due to presence of an inner incomplete d sub-shell • Electrons from both inner d sub-shell and outer s sub-shell can be involved in compound formation What is a transition metal? • Not all d block elements have incomplete d sub-shells – e.g. Zn has e.c. of [Ar]3d104s2, the Zn2+ ion ([Ar] 3d10) is not a typical TM ion – Similarly Sc forms Sc3+ which has the stable e.c of Ar. Sc3+ has no 3d electrons What is a transition metal? • For this reason, a transition metal is defined as being an element which forms at least one ion with a partially filled sub-shell of d electrons. – In period 4 only Ti-Cu are TM’s! – Note that when d block elements form ions the s electrons are lost first Tm complex: Variable valence Sc +3 Ti +1 +2 +3 +4 V +1 +2 +3 +4 +5 Cr +1 +2 +3 +4 +5 +6 Mn +1 +2 +3 +4 +5 +6 Fe +1 +2 +3 +4 +5 +6 Co +1 +2 +3 +4 +5 Ni +1 +2 +3 +4 Cu +1 +2 +3 Zn +2 +7 Cu is the only element which affords CuI compounds without acceptor ligands Complexes: Have metal ion (can be zero oxidation state) bonded to number of ligands. Complex contains central metal ion bonded to one or more molecules or anions Lewis acid = metal = center of coordination Transition metals can act as Lewis acid Lewis base = ligand = molecules/ions covalently bonded to metal in complex The term ligand (ligare [Latin], to bind) was first used by Alfred Stock in 1916 in relation to silicon chemistry. The first use of the term in a British journal was by H. Irving and R.J.P. Williams in Nature, 1948, 162, 746. For a fascinating review on 'ligand' in chemistry - Polyhedron, 2, 1983, 1-7. Ligand: Lewis base – contain at least one nonbonding pair of electrons Ni2+(aq) + 6NH3(aq) Ni(NH3)62+(aq) Lewis acid Lewis base Complex ion Coordination compound Compound that contains 1 or more complexes Example [Co(NH3)6]Cl3 [Cu(NH3)4][PtCl4] [Pt(NH3)2Cl2] Teeth of a ligand ( teeth dent) • Ligands – classified according to the number of donor atoms – Examples chelating agents • monodentate = 1 • • • • bidentate = 2 tetradentate = 4 hexadentate = 6 polydentate = 2 or more donor atoms monodentate, bidentate, tridentate etc. where the concept of teeth (dent) is introduced, hence the idea of bite angle etc. oxalate ion O O C C 2- ethylenediamine CH2 CH2 H2N O O * * * NH2 * Coordination Equilibria & Chelate effect "The adjective chelate, derived from the great claw or chela (chely - Greek) of the lobster, is suggested for the groups which function as two units and fasten to the central atom so as to produce heterocyclic rings." J. Chem. Soc., 1920, 117, 1456 Ni2+ The chelate effect or chelation is one of the most important ligand effects in transition metal coordination chemistry. Coordination Equilibria & Chelate effect [Fe(H2O)6]3+ + NCS- [Fe(H2O)5(NCS)]2+ + H2O Kf = [Fe(H2O)5(NCS)]2+/ [Fe(H2O)6]3+[NCS-] Equilibrium constant Kf formation constant M + L ML K1 = [ML]/[M][L] ML + L ML2 K2 = [ML2]/[ML][L] ML2 + L ML3 K3 = [ML3]/[ML2][L] MLn-1 + L MLn Kn = [MLn]/[MLn-1][L] Coordination Equilibria and Chelate effect • K1, K2…. Stepwise formation constant. • To calculate concentration of the final product, use overall formation constant n: • n = [MLn]/[M][L]n • = K1 x K2 x K3 x …. x Kn Coordination Equilibria & Chelate effect Example: [Cd(NH3)4]2+ Cd2+ + NH3 [CdNH3]2+ K1 = 102.65 [CdNH3]2+ + NH3 [Cd(NH3)2]2+ K2 = 102.10 [Cd(NH3)2]2++ NH3 [Cd(NH3)3]2+ K3 = 101.44 [Cd(NH3)3]2++ NH3 [Cd(NH3)4]2+ K4 = 100.93 Overall: Cd2+ + 4 NH3 [Cd(NH3)4]2+ β4 = K1 x K2 x K3 x K4 = 10(2.65 + 2.10 + 1.44 + 0.93) = 107.12 What are the implications of the following results? NiCl2 + 6H2O [Ni(H2O)6]+2 [Ni(H2O)6]+2 + 6NH3 [Ni(NH3)6]2+ + 6H2O log = 8.6 [Ni(H2O)6]+2 + 3 NH2CH2CH2NH2 (en) log = 18.3 [Ni(en)3]2+ + 6H2O [Ni(NH3)6]2+ + 3 NH2CH2CH2NH2 (en) [Ni(en)3]2+ + 6NH3 log = 9.7 Complex Formation: Major Factors [Ni(H2O)6] + 6NH3 [Ni(NH3)6]2+ + 6H2O NH3 is a stronger (better) ligand than H2O O NH3 > O H2O [Ni(NH3)6]2+ is more stable G = H - TS (H -ve, S 0) G for the reaction is negative Chelate Formation: Major Factors [Ni(NH3)6]2+ + 3 NH2CH2CH2NH2 (en) [Ni(en)3]2+ + 6NH3 en and NH3 have similar N-donor environment but en is bidentate and chelating ligand rxn proceeds towards right, G negative G = H - TS (H -ve, S ++ve) rxn proceeds due to entropy gain S ++ve is the major factor behind chelate effect Chelate Formation: Entropy Gain Cd2+ + 4 NH3 [Cd(NH3)4]2+ Cd2+ + 4 MeNH2 [Cd(MeNH2)4]2+ Cd2+ + 2 en [Cd(en)2]2+ Ligands log G kJmol-1 H kJmol-1 S JK-1mol-1 4 NH3 7.44 -42.5 - 53.2 - 35.5 4 MeNH2 6.52 -37.2 -57.3 - 67.3 2 en 10.62 -60.7 -56.5 +13.8 Chelate Formation: Entropy Gain Reaction of ammonia and en with Cu2+ [Cu(H2O)6]2+ + 2NH3 [Cu(NH3)2(H2O)2]2+ + 2 H2O Log 2 = 7.7 [Cu(H2O)6]2+ + en Log K1 = 10.6 H = -46 kJ/mol S = -8.4 J/K/mol [Cu(en)(H2O)4]2+ + 2 H2O H = -54 kJ/mol S = 23 J/K/mol Kinetic stability Inert and labile complexes The term inert and labile are relative “A good rule of thumb is that those complexes that react completely within 1 min at 25o should be considered labile and those that take longer should be considered inert.” Thermodynamically stable complexes can be labile or inert [Hg(CN)4]2- Kf= 1042 thermodynamically stable [Hg(CN)4]2- + 4 14CN- = [Hg(14CN)4]2- + CNVery fast reaction Labile Chelating agents: (1) Used to remove unwanted metal ions in water. (2) Selective removal of Hg2+ and Pb2+ from body when poisoned. (3) Prevent blood clots. (4) Solubilize iron in plant fertilizer. Important Chelating Ligands 2,3-dimercapto-1-propanesulfonic acid sodium (DMPS) Mn+ DMPS is a effective chelator with two groups thiols - for mercury, lead, tin, arsenic, silver and cadmium. SH O HO OH O SH (R,S)-2,3-dimercaptosuccinic acid D-Penicillamine As, Cu, Pb, Hg SH S M+ M HS OH Dimercaprol Zn As Hg Au Pb OH S As Hg Au Pb Important Chelating Ligands EDTA O *O C *O C CH2 * N O CH2 * CH2 CH2 N O CH2 C O* CH2 C O* O EDTA: another view Anticoagulant Ca2+ Important Chelating Ligands Macrocylic Ligands