* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Coccidioidomycosis: A review and update C ONTINUING MEDICAL EDUCATION
Tuberculosis wikipedia , lookup
Neglected tropical diseases wikipedia , lookup
West Nile fever wikipedia , lookup
Chagas disease wikipedia , lookup
Anaerobic infection wikipedia , lookup
Carbapenem-resistant enterobacteriaceae wikipedia , lookup
Gastroenteritis wikipedia , lookup
Eradication of infectious diseases wikipedia , lookup
Marburg virus disease wikipedia , lookup
Trichinosis wikipedia , lookup
Sexually transmitted infection wikipedia , lookup
Middle East respiratory syndrome wikipedia , lookup
Leptospirosis wikipedia , lookup
African trypanosomiasis wikipedia , lookup
Human cytomegalovirus wikipedia , lookup
Hepatitis C wikipedia , lookup
Onchocerciasis wikipedia , lookup
Leishmaniasis wikipedia , lookup
Hepatitis B wikipedia , lookup
Neonatal infection wikipedia , lookup
Sarcocystis wikipedia , lookup
Schistosomiasis wikipedia , lookup
Dirofilaria immitis wikipedia , lookup
Lymphocytic choriomeningitis wikipedia , lookup
Fasciolosis wikipedia , lookup
Oesophagostomum wikipedia , lookup
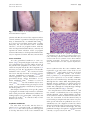
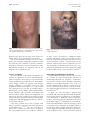
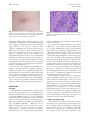
CONTINUING MEDICAL EDUCATION Coccidioidomycosis: A review and update David J. DiCaudo, MD Scottsdale, Arizona Coccidioidomycosis occurs in arid and semi-arid regions of the New World from the western United States to Argentina. Highly endemic areas are present in the southwest United States. Coccidioides species live in the soil and produce pulmonary infection via airborne arthroconidia. The skin may be involved by dissemination of the infection, or by reactive eruptions, such as a generalized exanthem or erythema nodosum. Interstitial granulomatous dermatitis and Sweet’s syndrome have recently been recognized as additional reactive signs of the infection. Coccidioidomycosis is a ‘‘great imitator’’ with protean manifestations. Cutaneous findings may be helpful clues in the diagnosis of this increasingly important disease. ( J Am Acad Dermatol 2006;55:929-42.) Learning objective: At the conclusion of this learning activity, participants should be familiar with the pathogenesis, clinical manifestations, risk factors, diagnosis, and treatment of coccidioidomycosis. C utaneous findings played an important role in the first reported case of coccidioidomycosis more than a century ago. In 1892, Posada1 described an Argentine soldier who had verrucous facial lesions, which clinically resembled mycosis fungoides. Distinctive organisms were visualized microscopically in the skin. Although initially believed to be a protozoan, Coccidiodes immitis was identified as a fungus in 1900.2 At that time, the disease was considered to be frequently fatal.3 The broad clinical spectrum of coccidioidomycosis was not appreciated until the 1930s, when C immitis was recognized as the cause of ‘‘valley fever’’ in the San Joaquin Valley of California.4 This common syndrome was a relatively mild, self-limited respiratory illness associated with erythema nodosum. Throughout the 20th century, as the desert southwest transformed into a popular destination for tourism and migration, coccidioidomycosis became an increasingly important and common disease.5 In recent decades, Coccidioides species have emerged as significant opportunistic pathogens in HIV patients6-15 and in organ transplant recipients16-23 in endemic areas. In the current era, there is growing concern that certain new biologic therapies may From the Departments of Dermatology and Pathology, Mayo Clinic. Funding sources: None. Conflict of interest: None identified. Reprint requests: David J. DiCaudo, MD, Mayo Clinic, 13400 E Shea Blvd, Scottsdale, AZ 85259. E-mail: [email protected]. 0190-9622/$32.00 ª 2006 by the American Academy of Dermatology, Inc. doi:10.1016/j.jaad.2006.04.039 predispose patients to an increased risk of severe coccidioidomycosis.24,25 The 21st century also brings the recognition of Coccidioides species as potential agents of bioterrorism.26,27 COCCIDIOIDES IMMITIS/POSADASII Coccidioides species are dimorphic fungi with saprophytic and parasitic phases (Fig 1).28 Humans, dogs, horses, and other animals may serve as hosts. The organisms’ life cycle explains several interesting characteristics of the disease. Coccidioidomycosis is infectious, but not contagious. Nearly all infections are acquired from the environment by the inhalation of airborne arthroconidia from the soil. In laboratory cultures, the organisms grow as the extremely infectious mycelial form, which may be hazardous to laboratory workers. The organisms are considered to be potential agents of bioterrorism.26,27 Because they are highly infectious and sporulate easily in culture, Coccidioides species are the only fungi on the Select Agent list of the Department of Health and Human Services.29 Federal regulations restrict the possession, use, and transfer of select biological agents. According to a recent review, however, terrorists would likely encounter significant difficulties in attempting to employ the organisms as effective weapons.27 The genus Coccidioides demonstrates genomic diversity.30,31 DNA polymorphisms distinguish the California strains of Coccidioides from the nonCalifornia strains.32 A separate species name (C posadasii) has recently been proposed for the non-California clade, while the original species name (C immitis) is reserved for the Californian clade.33 At the current time, the designation of two 929 930 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 Fig 1. Life cycle of Coccidioides immitis/posadasii. Filamentous mycelia grow in the soil and form arthroconidia. After dispersing into the air, arthroconidia may re-implant in the soil or may be inhaled by a host. If returned to the soil, arthroconidia grow into additional mycelia. If inhaled by a host, the arthroconidia transform into spherules in the lungs. Spherules produce and release endospores, which develop into additional spherules within the host tissues. The organism reverts to the mycelial form if it is grown in culture or if it is returned to the soil (for example, through a buried animal carcass). (Adapted from the diagram of Dr Hillel Levine in Dr David A. Stevens’ article28 with the permission of Drs Levine and Stevens.) species is controversial. Some researchers recognize only a single species, C immitis, and consider the non-California clade to be a variety (C immitis var posadasii). The clinical manifestations of coccidioidomycosis are generally similar regardless of geographic location. IMMUNE RESPONSE In coccidioidomycosis, cell-mediated immunity is important in establishing an effective response to the infection.34-37 The clinical course may be greatly influenced by whether the immune response is directed predominantly towards a TH1 pattern or towards a TH2 pattern.37 Interferon-gamma, a TH1associated cytokine, activates macrophages, which are subsequently able to inhibit or kill the organisms. In contrast, TH2-associated cytokines promote the production of antibodies and down-regulate TH1 functions. The antibodies do not appear to confer protection against the organisms. In self-limited infections, patients typically have strong delayed hypersensitivity responses to coccidioidal antigens and have low antibody titers. Conversely, patients with disseminated infection typically manifest weak or absent delayed hypersensitivity responses and have high antibody titers.37-39 An effective cell-mediated response provides resistance to re-infection. Following recovery from selflimited infections, patients typically have lifelong immunity.40 In addition to the T cellemediated mechanisms of acquired immunity, natural killer (NK) cells may play a role in innate resistance to the organism.37,41 A recent review provides a detailed discussion of innate and adaptive mechanisms of immunity in coccidioidomycosis.42 EPIDEMIOLOGY Incidence in endemic areas An estimated 100,000 cases of coccidioidomycosis occur annually in the United States.43 The geographic DiCaudo 931 J AM ACAD DERMATOL VOLUME 55, NUMBER 6 Fig 2. Geographic distribution of Coccidioides species. (Adapted from Kirkland et al,45 in the public domain.) distribution corresponds to regions with hot, dry summers, few winter freezes, low annual rainfall, and alkaline soil.43,44 Highly endemic areas include parts of Arizona and California (Fig 2).45 Major metropolitan areas with high incidence rates include Bakersfield, California; Phoenix, Arizona; and Tucson, Arizona. Confirmed cases are reportable in some states, including Arizona, California, and New Mexico. Skin test surveys generally suggest a downward trend in the incidence of coccidioidomycosis in the Bakersfield area over the past 60 years.46 This phenomenon is attributed to irrigation and urbanization of the region. Nevertheless, the same area witnessed a remarkable resurgence in the number of cases in the early 1990s. Climatic and demographic factors may have been responsible.45 Similarly, in Arizona, the incidence increased by more than 100% between 1990 and 1995,47,48 and by an additional 180% between 1995 and 2001.49 The incidence in Arizona has continued to increase, from 43 cases per 100,000 people in 2001 to 63/100,000 in 2004.50 Climatic and environmental factors Local climatic factors, including precipitation and temperature, influence the variable yearly incidence of coccidioidomycosis.44,45,51-53 Seasonal variations in incidence also occur. In Arizona, the number of new infections peaks during the winter months.49 In the western United States, epidemics have been associated with environmental events, such as dust storms,54 earthquakes,55,56 and droughts.51 An increased risk of infection is seen in persons with exposure to dust and soil. Outbreaks have occurred with occupational exposure in archeological workers57-60 and in military personnel.3,61-66 In the medical community, awareness of coccidioidomycosis is important even in non-endemic areas. Travelers who are exposed to the organism may develop symptoms after they return home.67-72 HOST FACTORS Genetic factors and co-morbid conditions may influence the host response to coccidioidal infection. The incidence of primary pulmonary coccidioidomycosis appears to be similar among different ethnic groups. However, specific groups, such as Filipinos and African Americans, have a markedly increased risk of severe disease and dissemination.73,74 HIV-infected persons,6-15,75-82 organ transplant recipients,16,17,19-22,83-85 other immunocompromised patients,23,86-92 and pregnant women82,93-97 also have an increased risk of severe disease and dissemination. Coccidioidomycosis is an AIDS-defining illness. Patients with CD4 counts less than 0.250 3 109/L have an increased risk of active coccidioidomycosis.8,10 In endemic areas, antiretroviral therapy appears to 932 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 have decreased the incidence of coccidioidomycosis in the HIV-infected population.9,13 By impairing cell-mediated immunity, immunosuppressive medications, such as prednisone and cyclosporine, may predispose to severe primary infection or may permit re-activation of the latent infection. Similar concerns may apply to tumor necrosis factor (TNF)ealpha antagonists, which are approved for the treatment of rheumatoid arthritis, ankylosing spondylitis, Crohn’s disease, and psoriasis. In a retrospective case series, coccidioidomycosis was documented in 13 patients receiving TNF-alpha antagonists (infliximab or etanercept) for inflammatory arthritis. Two of the patients, who were receiving both infliximab and methotrexate, died of disseminated coccidioidomycosis.25 In endemic areas, Crum et al24 recommend chest radiographs and serologies at baseline and every 3 to 4 months during treatment with TNF-alpha antagonists. Similar testing is recommended for patients who have previously resided in an endemic area.24 Further studies are needed to define the risk of severe coccidioidomycosis in patients receiving biologic therapies. CLINICAL FEATURES Approximately 60% of individuals with primary coccidioidomycosis are asymptomatic.3 Most of the remaining 40% experience mild to moderate influenza-like symptoms, including cough, fever, arthralgias, myalgias, and fatigue. In most patients, symptoms resolve or significantly improve within 2 to 3 weeks. Protective immunity follows recovery from the infection.40,98 Coccidioidomycosis occasionally produces prolonged morbidity or death. In a recent series of 223 patients, 2 died of respiratory failure. An additional 8 suffered chronic morbidity from disseminated involvement of the bones or meninges.74 The lungs are nearly always the primary focus of infection. Pulmonary manifestations may include pneumonia, pleural effusion, hilar lymphadenopathy, and lung nodules.43 Severe pulmonary involvement may occur, especially in immunocompromised patients. Rarely, a chronic progressive pneumonia may continue for years and produce pulmonary cavities and fibrosis. Disseminated infection occurs in approximately 1% of patients.3 Almost all patients with disseminated infection experience systemic symptoms, such as fever, cough, and night sweats.74 In rare cases of disseminated infection, there may be no clinical or radiographic evidence of a preceding respiratory illness.28 Dissemination may occur acutely with the primary pulmonary infection, or may be chronic and relapsing. Like Mycobacterium tuberculosis, C immitis/posadasii may be contained, rather than destroyed, by the immune response.99 In some cases, viable organisms may persist indefinitely within the tissues. If the host subsequently becomes immunocompromised, the quiescent infection may reactivate and disseminate. Common sites of disseminated coccidioidomycosis include the skin, meninges, bones, and joints. Coccidioidal meningitis is potentially lethal.100 Early manifestations of meningitis include headache, nausea, vomiting, and altered mental status. Nuchal rigidity and cranial neuropathies may occur. Dissemination to the bones and joints produces osteomyelitis and synovitis.101,102 Like tuberculosis and syphilis, coccidioidomycosis is a ‘‘great imitator’’ with protean manifestations.103,104 CUTANEOUS MANIFESTATIONS Cutaneous manifestations may be categorized as organism-specific or reactive.105 Organism-specific lesions contain the organisms, which may be identified in skin biopsy specimens by histopathologic examination or by culture. Organism-specific lesions result from hematogenous dissemination to the skin or, much more rarely, from a primary cutaneous infection. Reactive eruptions, which contain no viable organisms, include erythema nodosum, an acute generalized exanthem, erythema multiforme, Sweet’s syndrome, and reactive interstitial granulomatous dermatitis. Erythema nodosum Erythema nodosum (EN) is generally considered to be the most characteristic reactive cutaneous manifestation of coccidioidomycosis.106 One to three weeks after the onset of the illness,107 patients develop painful subcutaneous red nodules, typically on the lower extremities. The diagnosis of EN is frequently based upon the clinical features. If an excisional biopsy is performed, the characteristic histopathologic pattern is a septal granulomatous panniculitis. Other well-recognized causes of EN include sarcoidosis, streptococcal infection, other infections, inflammatory bowel disease, pregnancy, and oral contraceptives.108,109 Both ethnicity and gender appear to influence the incidence of EN in coccidioidomycosis.64,107 In a large study of military personnel from the early 1940s, EN developed in 50% of white women and in 18% of white men with the diagnosis of coccidioidomycosis.3 EN appears to be rare in African American men with the infection.3,64,107 EN may reflect the presence of a vigorous cellmediated immune response, which may confer a protective advantage against the organism.95 In J AM ACAD DERMATOL DiCaudo 933 VOLUME 55, NUMBER 6 Fig 3. Acute exanthem of coccidioidomycosis. A generalized morbilliform eruption. patients with EN, the onset of the eruption tends to coincide with the acquisition of delayed hypersensitivity, as determined by coccidioidal skin testing.107 In a report of 61 pregnant women with coccidioidomycosis, EN was associated with a favorable outcome.93 Of the 30 pregnant women with EN, none developed disseminated disease, and only one experienced chronic infection. Of the 31 pregnant women without EN, 11 developed disseminated disease, 11 experienced chronic infection, and one died. Acute exanthem An acute generalized exanthem or ‘‘toxic erythema’’ (Fig 3) frequently begins early in the course of coccidioidomycosis, typically within 48 hours of the patient’s first symptoms.110 In some cases, the cutaneous eruption may be the chief complaint111,112 and may precede the development of detectable antibodies in the serum.111 The cutaneous lesions have been clinically described as macular, papular, urticarial, morbilliform, or target-like.63,111,113 The clinical appearance may be mistaken for severe, generalized allergic contact dermatitis112 or erythema multiforme (EM).111 Pruritus is severe in some cases.64,111,113 An oral enanthem may be associated.110,114 The exanthem may persist up to several weeks, and occasionally is followed by desquamation of the palms.111 Skin biopsies demonstrate a non-specific pattern of spongiotic dermatitis and/or interface dermatitis with a mild perivascular inflammatory infiltrate including lymphocytes, neutrophils, eosinophils, and karyorrhectic debris.111 In contrast to EM, necrotic keratinocytes are rare. Erythema multiforme For more than six decades, EM has been reported to be associated with coccidioidomycosis.63,107,110,113 The clinical spectrum of EM appears to overlap with those of the acute exanthem and Fig 4. Sweet’s syndrome in a patient with pulmonary coccidioidomycosis. A, Annular vesiculated plaques clinically mimicking erythema multiforme. B, Skin biopsy consistent with Sweet’s syndrome. Dense dermal infiltrate including neutrophils, macrophages, and karyorrhectic debris (Hematoxylin-eosin stain; original magnification: 3600). Sweet’s syndrome. Like the acute exanthem, EM is reported to occur early in the course of the coccidioidomycosis,113 often within 48 hours of the first symptoms.110 Target-like lesions, oral involvement,110 pruritus,111,113 and palmar desquamation111 have been reported to occur in both conditions. Thus, the acute exanthem may clinically mimic EM, although the two entities differ histopathologically. Similarly, Sweet’s syndrome (see below) may have annular or target-like features which clinically resemble EM, although the histopathologic findings are extremely different (Fig 4, A and B). In a comprehensive review of EM, Huff et al115 did not consider coccidioidomycosis to be among the well-documented causes. While EM-like eruptions are described in numerous different infectious diseases, the association is well documented only in recurrent herpes simplex infection and in very few other specific infections. In published cases of coccidioidomycosis, the diagnosis of EM has almost always been based solely upon the clinical features. To the best of this author’s knowledge, the only published histologic description116 would not fulfill currently accepted criteria for EM.117,118 Necrotic 934 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 Fig 5. Reactive interstitial granulomatous dermatitis associated with pulmonary coccidioidomycosis. Firm coalescent papules and plaques on the knee. Fig 6. Disseminated coccidioidomycosis. Ulcerated frambesiform papules. keratinocytes, which are the most characteristic histologic feature, were lacking in the description. It is unclear whether true EM occurs in coccidioidomycosis, or whether the EM-like eruptions actually represent the acute exanthem or Sweet’s syndrome. Regardless of whether or not the eruptions are truly EM, EM-like eruptions are important clues to the diagnosis of coccidioidomycosis. in 2004, I have encountered 5 additional similar patients. Skin lesions resolve as the patient recovers from the underlying pulmonary infection. In most clinical situations, systemic corticosteroids are the mainstay of therapy for Sweet’s syndrome. In cases associated with coccidioidomycosis, however, recognition of the pulmonary infection is important so that treatment with systemic corticosteroids is avoided.127 Sweet’s syndrome Sweet’s syndrome (acute febrile neutrophilic dermatosis) is a distinctive, reactive, immunologicallyinduced eruption, which may be associated with a variety of underlying systemic diseases. Well-demarcated, boggy, tender red papules and plaques develop abruptly in association with fever and peripheral blood leukocytosis.119,120 The lesions may sometimes have vesicular or pustular features. Skin biopsies reveal a diffuse, dense dermal inflammatory infiltrate with numerous neutrophils and leukocytoclastic debris, but no microorganisms are detected in the skin. Common associations include nonspecific upper respiratory infections, hematologic malignancies, inflammatory bowel diseases, and connective tissue diseases.121-125 Only three patients have been reported with Sweet’s syndrome associated with acute pulmonary coccidioidomycosis.126,127 Yet, the association is likely more common than has been recognized. Since the publication of 2 cases with my coworkers Interstitial granulomatous dermatitis Granulomatous dermatitis may be seen not only in a variety of cutaneous infections, but also in immunologically-induced eruptions. Granulomatous tissue reactions, which resemble granuloma annulare or necrobiosis lipoidica, may occur as a reactive manifestation of diverse systemic diseases, such as connective tissue diseases, systemic vasculitis, lymphoma, infectious diseases, and inflammatory bowel disease.128 A single case series described 5 patients who presented with interstitial granulomatous dermatitis in association with acute pulmonary coccidioidomycosis. Edematous or indurated cutaneous papules, nodules, and plaques develop abruptly at the onset of the illness (Fig 5).105 Skin biopsies reveal interstitial dermal inflammation with macrophages, often accompanied by eosinophils, neutrophils, and leukocytoclastic debris. Fungal stains and cultures reveal no organisms within the skin biopsy specimens. In the published series, cutaneous lesions resolved J AM ACAD DERMATOL DiCaudo 935 VOLUME 55, NUMBER 6 Fig 7. Chronic disseminated coccidioidomycosis resembling lupus vulgaris. Despite previous systemic antifungal therapy, the plaque on the cheek has persisted for 10 years. over 1 week to 2 months, as the patients recovered from their respiratory symptoms. Since publication of the 5 cases with my coworker in 2001, I have encountered similar biopsy findings in 3 additional patients with serologically confirmed coccidioidomycosis. Further observation has revealed that the neutrophilic component of the infiltrate may vary greatly. Neutrophil-rich cases may lie on a continuum with Sweet’s syndrome. In other cases, the dermis may contain only a sparse collection of interstitial macrophages with rare neutrophils. Nevertheless, the histopathologic pattern is often distinctive and may raise suspicion for coccidioidomycosis, even in the absence of clinical history. Interstitial granulomatous dermatitis and Sweet’s syndrome may clinically and histopathologically resemble a disseminated infection. Skin biopsy specimens should be evaluated by culture and by histopathologic examination, in order to distinguish these two reactive eruptions from a disseminated infection. Disseminated infection The skin is the most common site of disseminated coccidioidomycosis.43,129 When dissemination occurs, it usually begins within several weeks or months after the primary infection,3 but occasionally it may be the initial manifestation.130 Almost all disseminated cases arise from a primary pulmonary infection, which spreads hematogenously to the skin. A variety of cutaneous lesions may occur, including papules, nodules, verrucous plaques, abscesses, pustules, and sinus tracts.129,131 Skin lesions may be solitary or multiple and may ulcerate (Fig 6). Even after resolution of other symptoms, disseminated skin lesions may rarely become chronic (Figs 7 and 8). The diverse presentations include unusual cases that clinically resemble tumor-stage mycosis fungoides1,74 or lepromatous leprosy.132 Fig 8. Chronic disseminated coccidioidomycosis of 5 years’ duration. Tumid, indurated plaques on the cheeks, nose, and upper lip. In disseminated coccidioidomycosis, skin biopsies reveal granulomatous and/or suppurative inflammation in the dermis or subcutaneous adipose tissue. The inflammatory infiltrate often includes numerous eosinophils.131 The overlying epidermis may show pseudocarcinomatous hyperplasia with or without ulceration. Organisms are identified by histopathologic examination and by cultures of skin biopsy specimens, although either method may sometimes yield falsely negative results.131 If the organism is identified in the skin, other possible sites of dissemination should be investigated.43,130,133 Primary cutaneous infection Only approximately 20 cases of primary cutaneous coccidioidomycosis have been reported in the literature.134-142 A comprehensive review of the subject has recently been published.141 Traumatic inoculation of the organism results from direct contact with a source in the environment or in the laboratory. Primary cutaneous infections have been reported in agricultural workers,136 laboratory workers,137,139,142 an embalmer,140 and in persons suffering splinter injuries or lacerations.134,135,138 Primary cutaneous coccidioidomycosis typically manifests as an ulcerated nodule on an extremity, although other sites may also be affected (Fig 9). In a pattern resembling sporotrichosis, secondary nodules sometimes arise in a linear distribution along the lymphatic pathways of an extremity.135 Fever and regional lymphadenopathy may be associated, and the affected lymph nodes may ulcerate. Though primary cutaneous infections sometimes resolve spontaneously, at least one patient experienced a severe clinical course with meningeal dissemination.135 In some cases, a primary pulmonary infection may disseminate to a single focus in the skin and may mimic a primary cutaneous infection. If the patient’s 936 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 Fig 9. Primary cutaneous coccidioidomycosis resulting from a cactus spine injury on the abdomen. After initial treatment with excision and systemic antifungal therapy, the infection recurred within the surgical scar. pulmonary symptoms are minimal or absent, it may be difficult to distinguish a disseminated infection from a primary cutaneous infection. The histopathologic features in the skin are similar in both instances. Organisms may be detected by histopathology or by culture in both situations. Even a history of localized trauma to the affected area does not completely rule out the possibility of a disseminated infection. In some cases, disseminated organisms in the bloodstream may specifically localize to the site of an injury (blunt trauma, laceration, or surgical wound). This phenomenon, termed locus minoris resistentiae, is well documented in disseminated coccidioidomycosis.143 Several clues may support the possibility of a primary cutaneous infection. Regional lymphadenopathy, a relatively low complement fixation titer, and a history of a trauma-induced break in the skin are all typical of primary cutaneous infection.143 Nevertheless, these findings are not entirely specific. In some cases of isolated cutaneous coccidioidomycosis, it may not be possible to distinguish primary cutaneous infection from a disseminated infection. DIAGNOSIS Serology Both qualitative and quantitative serologic tests are useful in the diagnosis of coccidioidomycosis. Enzyme immunoassay, latex particle agglutination, and immunodiffusion may be used as qualitative techniques, which yield positive results early in the course of the infection. Enzyme immunoassay provides rapid qualitative assessment of both IgM and IgG coccidioidal antibodies.144 While this test is highly sensitive, false positive reactions do sometimes occur.98,145 Positive reactions may require confirmation by another more specific technique. IgM antibodies occur mostly during early primary Fig 10. Coccidioidal spherule within a giant cell in the papillary dermis (Hematoxylin-eosin stain; original magnification: 3600). infection, although occasionally they may persist in chronic infections.98,145 IgG complement-fixing antibodies may appear within 2 to 3 weeks of the onset of symptoms, and are assessed quantitatively in the serum and other body fluids by quantitative immunodiffusion or complement fixation techniques.98 These techniques yield highly specific results.145 Even low titers of complement-fixing antibodies (1:2) are likely to signify a true infection. Complement-fixing antibodies usually disappear as the infection resolves but may persist in chronic infections. The titer generally correlates with the severity of the disease. Patients with disseminated infections typically have high titers (1:16, 1:32, or 1:64, depending upon the individual laboratory).98 However, some patients with limited dissemination to the skin may have lower titers, such as 1:4 or 1:8.98 Quantitative serologies are also useful in monitoring response to therapy. False negative serologic results do sometimes occur, particularly early in the course of infection. Some cutaneous manifestations, such as the acute exanthem, occur within the first 48 hours of the illness, and may precede the development of detectable antibodies. If clinically indicated, serologic testing may be repeated once or twice over the following 2 months, in order to document seroconversion.146 Persistently false negative serologies may occur in a small percentage of HIV-infected patients, other immunosuppressed patients, and even occasionally in immunocompetent individuals.98 Culture and microscopy The organism may be detected by culture of tissue specimens on most mycologic or bacteriologic media.146 Growth is rapid, occurring in as early as 2 days to more than 5 days.43 The colonies are typically white and cottony, but the morphology and color are variable.146 The microscopic appearance also DiCaudo 937 J AM ACAD DERMATOL VOLUME 55, NUMBER 6 is nonspecific in cultured specimens. Arthroconidia are typically barrel-shaped. The cultured organism is specifically identified by DNA probes or by detection of specific coccidioidal exoantigens.147-149 When specimens are submitted for culture, the laboratory should be appropriately notified because of the potential danger to laboratory workers. Direct microscopic visualization provides another means of identification. In tissue specimens and in cytologic smears, the spherules may be visualized with fungal stains or with a routine hematoxylin-eosin stain (Fig 10). The thick-walled spherules are usually distinguishable from other parasitic fungi by their relatively large size (10 to 80 microns). Characteristic endospores are frequently seen within the spherules. Molecular techniques Molecular techniques, such as in situ hybridization (ISH) and polymerase chain reaction (PCR), may assist in the identification of C immitis/posadasii in paraffin-embedded tissue. ISH is less sensitive but more specific than standard histochemical stains, such as methenamine silver stain.150 In cases where the visualized organisms are not morphologically distinctive, ISH may provide specific confirmation in histologic sections. This technique may be particularly useful in distinguishing small, immature spherules of C immitis/posadasii from Blastomyces dermatitidis and Cryptococcus neoformans.150 It should be noted that the spherules generally must be visible with standard histochemical stains, in order for ISH to be helpful. In addition to ISH techniques, PCR assays have recently been described for identification of C posadasii DNA in paraffinembedded specimens.151 Skin testing Delayed hypersensitivity skin tests for coccidioidal antigens are useful in population studies, which evaluate overall trends in the incidence of coccidioidomycosis. However, skin testing is of limited usefulness in the diagnosis of an acute infection. A positive skin test does not distinguish current infection from prior infection. In addition, anergy may develop during active infection in some cases. Individuals with reactive skin tests often show waning in reactivity after approximately 12 to 15 years.46 Currently, the delayed hypersensitivity skin tests for coccidioidal antigens are not generally available for clinical use in the United States. Imaging studies In acute symptomatic pulmonary coccidioidomycosis, chest radiographs demonstrate parenchymal abnormalities in almost 75% of cases.152 Segmental pneumonia is the most common pattern. Other common findings include single or multiple pulmonary nodules, hilar or mediastinal lymphadenopathy, and pleural effusions. The findings are not specific and may mimic bacterial pneumonia, tuberculosis, or a malignancy.153 Approximately 5% of patients will develop chronic abnormalities, such as pulmonary cavities or persistent nodules.153 In disseminated infections, chest radiographs may reveal a reticulonodular or diffuse military pattern. In complicated or disseminated infections, other imaging modalities may be indicated in addition to standard chest radiographs.153,154 Computed tomography of the lungs and central nervous system may be helpful in individual cases.154-157 Scintigraphy provides a useful screening tool when disseminated skeletal involvement is suspected.158 With plain radiographs, skeletal lesions usually have a lytic, ‘‘punched out’’ appearance. Nearly any bone can be affected, but the axial skeleton is most frequently involved.159 Computed tomography and magnetic resonance imaging may be useful in evaluating involvement of the soft tissues and spine.159 TREATMENT The therapeutic approach to coccidioidomycosis is based predominantly upon three factors: (1) the severity of the pulmonary infection, (2) the presence or absence of dissemination, and (3) the individual patient’s risk factors.160,161 Treatment guidelines have recently been published160,162 and are also currently available online at the Infectious Diseases Society of America Web site (http://www.IDSociety. org) under the heading of Practice Guidelines. In immunocompetent patients with primary uncomplicated pulmonary infection, antifungal therapy is controversial. Prospective controlled trials have not been performed. There is no evidence that treatment of the primary infection mitigates the severity of the illness or prevents complications.43 For most patients with a mild respiratory illness, many authorities believe that antifungal therapy is not necessary.43 Patients should be periodically evaluated clinically and radiographically for one to two years, in order to document resolution of the infection. On the other hand, some experts advocate systemic antifungal therapy for all symptomatic patients. Either itraconazole 200 mg twice daily or fluconazole 400 mg/d may be given by mouth for approximately 3 to 6 months. If risk factors for dissemination are present, antifungal therapy should be given.160,162,163 HIV patients, allograft recipients, and other immunosuppressed patients may be treated with systemic antifungal agents. Specific ethnic backgrounds, such as 938 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 Filipino or African American ancestry, may also be considered a risk factor for dissemination. Regardless of the patient’s ethnicity, antifungal therapy may be strongly considered for symptomatic pregnant patients (particularly during the third trimester) and in the post-partum period. During pregnancy, amphotericin B is the treatment of choice. In pregnant women, the azoles are contraindicated because of the risk of teratogenicity. Prophylactic therapy may have a role in the care of some immunosuppressed patients, who have a high risk of developing coccidioidomycosis. Targeted azole chemoprophylaxis may be considered for specific groups of organ transplant recipients and HIV patients.9,21,164 Antifungal therapy is indicated in patients with disseminated infection or severe pulmonary infection. Treatment recommendations for severe infections are included in the recently published comprehensive guidelines160,162 and are beyond the scope of this review. After initial treatment of disseminated infection, lifelong therapy with azoles may be required in some cases. Azoles are fungistatic, rather than fungicidal. Recurrences are common after discontinuing therapy. In the future, a variety of new antifungal medications may prove to be useful in the treatment of coccidioidomycosis.43,165-173 Efforts to develop a vaccine are in progress.42,174-180 CONCLUSION Coccidioidomycosis is a ‘‘great imitator’’ with protean clinical manifestations. Knowledge of the diverse cutaneous clues can be helpful in the diagnosis of this increasingly important disease. The author is grateful for the assistance of Ms Aimee Volner in the Section of Illustration & Design at Mayo Clinic in Rochester, Minnesota. Ms Volner designed the adaptions of the diagrams in Figs 1 and 2. The author’s colleague Dr Joseph P. Fiore is thanked for contributing the cases shown in Figs 7 and 8. REFERENCES 1. Posada A. Un nuevo caso de micosis fungoidea con psorospermias. An Circ Med Argent 1892;15:585-97. 2. Ophuls W, Moffitt H. A new pathogenic mould formerly described as a protozoan (Coccidioides immitis pyogenes): preliminary report. Philadelphia Med 1900;J5:1471-2. 3. Smith C, Beard R, Whiting E, Rosenberger H. Varieties of coccidiodal infection in relation to the epidemiology and control of the diseases. Am J Public Health 1946;36:1394-402. 4. Dickson E. ‘‘Valley fever’’ of the San Joaquin Valley and fungus Coccidioides. Calif West Med 1937;47:151-7. 5. Galgiani JN. Coccidioidomycosis: a regional disease of national importance. Rethinking approaches for control. Ann Intern Med 1999;130:293-300. 6. Galgiani JN, Ampel NM. Coccidioides immitis in patients with human immunodeficiency virus infections. Semin Respir Infect 1990;5:151-4. 7. Galgiani JN, Ampel NM. Coccidioidomycosis in human immunodeficiency virus-infected patients. J Infect Dis 1990;162: 1165-9. 8. Fish DG, Ampel NM, Galgiani JN, Dols CL, Kelly PC, Johnson CH, et al. Coccidioidomycosis during human immunodeficiency virus infection. A review of 77 patients. Medicine 1990;69:384-91. 9. Woods CW, McRill C, Plikaytis BD, Rosenstein NE, Mosley D, Boyd D, et al. Coccidioidomycosis in human immunodeficiency virus-infected persons in Arizona, 1994-1997: incidence, risk factors, and prevention. J Infect Dis 2000;181: 1428-34. 10. Singh VR, Smith DK, Lawerence J, Kelly PC, Thomas AR, Spitz B, et al. Coccidioidomycosis in patients infected with human immunodeficiency virus: review of 91 cases at a single institution. Clin Infect Dis 1996;23:563-8. 11. Ampel NM, Dols CL, Galgiani JN. Coccidioidomycosis during human immunodeficiency virus infection: results of a prospective study in a coccidioidal endemic area. Am J Med 1993;94:235-40. 12. Sarosi GA, Davies SF. Endemic mycosis complicating human immunodeficiency virus infection. West J Med 1996;164: 335-40. 13. Ampel NM. Coccidioidomycosis among persons with human immunodeficiency virus infection in the era of highly active antiretroviral therapy (HAART). Semin Respir Infect 2001;16: 257-62. 14. Jones JL, Fleming PL, Ciesielski CA, Hu DJ, Kaplan JE, Ward JW. Coccidioidomycosis among persons with AIDS in the United States. J Infect Dis 1995;171:961-6. 15. Prichard JG, Sorotzkin RA, James RE 3rd. Cutaneous manifestations of disseminated coccidioidomycosis in the acquired immunodeficiency syndrome. Cutis 1987;39:203-5. 16. Blair JE, Logan JL. Coccidioidomycosis in solid organ transplantation. Clin Infect Dis 2001;33:1536-44. 17. Logan JL, Blair JE, Galgiani JN. Coccidioidomycosis complicating solid organ transplantation. Semin Respir Infect 2001;16:251-6. 18. Cohen IM, Galgiani JN, Potter D, Ogden DA. Coccidioidomycosis in renal replacement therapy. Arch Intern Med 1982; 142:489-94. 19. Schroter GP, Bakshandeh K, Husberg BS, Well R 3rd. Coccidioidomycosis and renal transplantation. Transplantation 1977; 23:485-9. 20. Hall KA, Sethi GK, Rosado LJ, Martinez JD, Huston CL, Copeland JG. Coccidioidomycosis and heart transplantation. J Heart Lung Transplant 1993;12:525-6. 21. Holt CD, Winston DJ, Kubak B, Imagawa DK, Martin P, Goldstein L, et al. Coccidioidomycosis in liver transplant patients. Clin Infect Dis 1997;24:216-21. 22. Riley DK, Galgiani JN, O’Donnell MR, Ito JI, Beatty PG, Evans TG. Coccidioidomycosis in bone marrow transplant recipients. Transplantation 1993;56:1531-3. 23. Murphey SM, Drash AL, Donnelly WM. Disseminated coccidioidomycosis associated with immunosuppressive therapy following renal transplantation. Pediatrics 1971;48:144-5. 24. Crum NF. Infections associated with tumor necrosis factor[alpha] antagonists. Arthritis Rheum 2005;84:291-302. 25. Bergstrom L, Yocum DE, Ampel NM, Villanueva I, Lisse J, Gluck O, et al. Increased risk of coccidioidomycosis in patients treated with tumor necrosis factor alpha antagonists. Arthritis Rheum 2004;50:1959-66. 26. Dixon DM. Coccidioides immitis as a Select Agent of bioterrorism. J Appl Microbiol 2001;91:602-5. J AM ACAD DERMATOL DiCaudo 939 VOLUME 55, NUMBER 6 27. Deresinski S. Coccidioides immitis as a potential bioweapon. Semin Respir Infect 2003;18:216-9. 28. Stevens DA. Coccidioidomycosis. New Engl J Med 1995;332: 1077-82. 29. Possession, use, and transfer of select agents and toxins; interim final rule. Vol 42 C.F.R §73.4-73.5; 2002. 30. Burt A, Dechairo BM, Koenig GL, Carter DA, White TJ, Taylor JW. Molecular markers reveal differentiation among isolates of Coccidioides immitis from California, Arizona and Texas. Mol Ecol 1997;6:781-6. 31. Koufopanou V, Burt A, Taylor JW. Concordance of gene genealogies reveals reproductive isolation in the pathogenic fungus Coccidioides immitis. Proc Natl Acad Sci U S A 1997; 94:5478-82. 32. Fisher MC, Rannala B, Chaturvedi V, Taylor JW. Disease surveillance in recombining pathogens: multilocus genotypes identify sources of human Coccidioides infections. Proc Natl Acad Sci U S A 2002;99:9067-71. 33. Fisher MC, Koenig GL, White TJ, Taylor JW. Molecular and phenotypic description of Coccidioides posadasii sp. nov., previously recognized as the non-California population of Coccidioides immitis. Mycologia 2002;94:73-84. 34. Ampel NM, Christian L. In vitro modulation of proliferation and cytokine production by human peripheral blood mononuclear cells from subjects with various forms of coccidioidomycosis. Infect Immun 1997;65:4483-7. 35. Ampel NM, Christian L. Flow cytometric assessment of human peripheral blood mononuclear cells in response to a coccidioidal antigen. Med Mycol 2000;38:127-32. 36. Ampel NM, Kramer LA. In vitro modulation of cytokine production by lymphocytes in human coccidioidomycosis. Cell Immunol 2003;221:115-21. 37. Cox RA, Magee DM. Protective immunity in coccidioidomycosis. Res Immunol 1998;149:417-28. 38. Cox RA, Vivas JR. Spectrum of in vivo and in vitro cellmediated immune responses in coccidioidomycosis. Cell Immunol 1977;31:130-41. 39. Smith CE, Saito MT, Simons SA. Pattern of 39,500 serologic tests in coccidioidomycosis. JAMA 1956;160:546-52. 40. Smith C, Pappagianis D, Levine H, Saito M. Human coccidioidomycosis. Bacteriol Rev 1961;25:310-20. 41. Petkus AF, Baum LL. Natural killer cell inhibition of young spherules and endospores of Coccidioides immitis. J Immunol 1987;139:3107-11. 42. Cox RA, Magee DM. Coccidioidomycosis: host response and vaccine development. Clin Microbiol Rev 2004;17:804-39. 43. Chiller TM, Galgiani JN, Stevens DA. Coccidioidomycosis. Infect Dis Clin North Am 2003;17:41-57. 44. Kolivras KN, Comrie AC. Modeling valley fever (coccidioidomycosis) incidence on the basis of climate conditions. Int J Biometeorol 2003;47:87-101. 45. Kirkland TN, Fierer J. Coccidioidomycosis: a reemerging infectious disease. Emerg Infect Dis 1996;2:192-9. 46. Larwood TR. Coccidioidin skin testing in Kern County, California: decrease in infection rate over 58 years. Clin Infect Dis 2000;30:612-3. 47. Centers for Disease Control and Prevention. Coccidioidomycosis—Arizona, 1990-1995. MMWR Morb Mortal Wkly Rep 1996;45:1069-73. 48. Ampel NM, Mosley DG, England B, Vertz PD, Komatsu K, Hajjeh RA. Coccidioidomycosis in Arizona: increase in incidence from 1990 to 1995. Clin Infect Dis 1998;27:1528-30. 49. Centers for Disease Control and Prevention. Increase in coccidioidomycosis—Arizona, 1998-2001. MMWR Morb Mortal Wkly Rep 2003;52:109-12. 50. Arizona Department of Health Services. Bureau of Epidemiology and Disease Control Services. Rate per 100,000 population of reported cases of selected notifiable diseases by category, Arizona, 1994-2004. Available at: http://www.azdhs. gov/phs/oids/stats/pdf/Rates1994-2004.pdf. Accessed April 5, 2006. 51. Fisher MC, Koenig GL, White TJ, Taylor JW. Pathogenic clones versus environmentally driven population increase: analysis of an epidemic of the human fungal pathogen Coccidioides immitis. J Clin Microbiol 2000;38:807-13. 52. Park BJ, Sigel K, Vaz V, Komatsu K, McRill C, Phelan M, et al. An epidemic of coccidioidomycosis in Arizona associated with climatic changes, 1998-2001. J Infect Dis 2005;191: 1981-7. 53. Comrie AC. Climate factors influencing coccidioidomycosis seasonality and outbreaks. Environ Health Perspect 2005; 113:688-92. 54. Flynn NM, Hoeprich PD, Kawachi MM, Lee KK, Lawrence RM, Goldstein E, et al. An unusual outbreak of windborne coccidioidomycosis. N Engl J Med 1979;301:358-61. 55. Schneider E, Hajjeh RA, Spiegel RA, Jibson RW, Harp EL, Marshall GA, et al. A coccidioidomycosis outbreak following the Northridge, Calif, earthquake. JAMA 1997;277:904-8. 56. Centers for Disease Control and Prevention. Coccidioidomycosis following the Northridge Earthquake—California, 1994. MMWR Morb Mortal Wkly Rep 1994;43:194-5. 57. Centers for Disease Control and Prevention. Coccidioidomycosis in workers at an archeologic site—Dinosaur National Monument, Utah, June-July 2001. MMWR Morb Mortal Wkly Rep 2001;50:1005-8. 58. Petersen LR, Marshall SL, Barton-Dickson C, Hajjeh RA, Lindsley MD, Warnok DW, et al. Coccidioidomycosis among workers at an archeological site, northeastern Utah. Emerg Infect Dis 2004;10:637-42. 59. Werner SB, Pappagianis D, Heindl I, Mickel A. An epidemic of coccidioidomycosis among archeology students in northern California. N Engl J Med 1972;286:507-12. 60. Werner SB, Pappagianis D. Coccidioidomycosis in Northern California. An outbreak among archeology students near Red Bluff. Calif Med 1973;119:16-20. 61. Crum N, Lamb C, Utz G, Amundson D, Wallace M. Coccidioidomycosis outbreak among United States Navy SEALs training in a Coccidioides immitiseendemic area—Coalinga, California. J Infect Dis 2002;186:865-8. 62. Crum NF, Lederman ER, Hale BR, Lim ML, Wallace MR. A cluster of disseminated coccidioidomycosis cases at a US military hospital. Mil Med 2003;168:460-4. 63. Goldstein D, McDonald J. Primary pulmonary coccidioidomycosis. Follow-up of 75 cases, with 10 more cases from a new endemic area. JAMA 1944;124:557-61. 64. Willett FM, Weiss A. Coccididioidomycosis in Southern California: report of a new endemic area with a review of 100 cases. Ann Int Med 1945;23:349-75. 65. Olivere JW, Meier PA, Fraser SL, Morrison WB, Parsons TW, Drehner DM. Coccidioidomycosis—the airborne assault continues: an unusual presentation with a review of the history, epidemiology, and military relevance. Aviat Space Environ Med 1999;70:790-6. 66. Standaert SM, Schaffner W, Galgiani JN, Pinner RW, Kaufman L, Durry E, et al. Coccidioidomycosis among visitors to a Coccidioides immitiseendemic area: an outbreak in a military reserve unit. J Infect Dis 1995;171:1672-5. 67. Panackal AA, Hajjeh RA, Cetron MS, Warnock DW. Fungal infections among returning travelers. Clin Infect Dis 2002; 35:1088-95. 940 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 68. Desai SA, Minai OA, Gordon SM, O’Neil B, Wiedemann HP, Arroliga AC. Coccidioidomycosis in non-endemic areas: a case series. Respir Med 2001;95:305-9. 69. Chaturvedi V, Ramani R, Gromadzki S, Rodeghier B, Chang HG, Morse DL. Coccidioidomycosis in New York State. Emerg Infect Dis 2000;6:25-9. 70. Centers for Disease Control and Prevention. Coccidioidomycosis in travelers returning from Mexico—Pennsylvania. MMWR Morb Mortal Wkly Rep 2000;49:1004-6. 71. Cairns L, Blythe D, Kao A, Pappagianis D, Kaufman L, Kobayashi J, et al. Outbreak of coccidioidomycosis in Washington state residents returning from Mexico. Clin Infect Dis 2000;30:61-4. 72. Long JB, Brett AS, Horvath JA. Coccidioidomycosis diagnosed in South Carolina. South Med J 2005;98:930-2. 73. Einstein HE, Johnson RH. Coccidioidomycosis: new aspects of epidemiology and therapy. Clin Infect Dis 1993;16:349-54. 74. Crum NF, Lederman ER, Stafford CM, Parrish JS, Wallace MR, Chang A, et al. Coccidioidomycosis: a descriptive survey of a reemerging disease. Clinical characteristics and current controversies. Medicine 2004;83:149-75. 75. Ampel NM. Delayed-type hypersensitivity, in vitro T-cell responsiveness and risk of active coccidioidomycosis among HIV-infected patients living in the coccidioidal endemic area. Med Mycol 1999;37:245-50. 76. Ampel NM. Emerging disease issues and fungal pathogens associated with HIV infection. Emerg Infect Dis 1996;2:109-16. 77. Antoniskis D, Larsen RA, Akil B, Rarick MU, Leedom JM. Seronegative disseminated coccidioidomycosis in patients with HIV infection. AIDS 1990;4:691-3. 78. Cockerell CJ. Cutaneous fungal infections in HIV/AIDS. J Int Assoc Physicians AIDS Care 1995;1:19-23. 79. Johnson RA. HIV disease: mucocutaneous fungal infections in HIV disease. Clin Dermatol 2000;18:411-22. 80. Kappe R, Levitz S, Harrison TS, Ruhnke M, Ampel NM, JustNubling G. Recent advances in cryptococcosis, candidiasis and coccidioidomycosis complicating HIV infection. Med Mycol 1998;36:207-15. 81. McNeil MM, Ampel NM. Opportunistic coccidioidomycosis in patients infected with human immunodeficiency virus: prevention issues and priorities. Clin Infect Dis 1995;21:S111-3. 82. Calderon-Garciduenas AL, Pina-Osuna K, Leal-Moreno AM, Lopez-Cardenas A, Cerda-Flores RM. Caracteristicas clinicopatologicas y distribucion del numero de autopsias de pacientes fallecidos por coccidioidomicosis en un hospital de referencia del noreste de Mexico. Gac Med Mex 2004; 140:399-404. 83. Cha JM, Jung S, Bahng HS, Lim CM, Han DJ, Woo JH, et al. Multi-organ failure caused by reactivated coccidioidomycosis without dissemination in a patient with renal transplantation. Respirology 2000;5:87-90. 84. Hall KA, Copeland JG, Zukoski CF, Sethi GK, Galgiani JN. Markers of coccidioidomycosis before cardiac or renal transplantation and the risk of recurrent infection. Transplantation 1993;55:1422-4. 85. Blair JE, Douglas DD. Coccidioidomycosis in liver transplant recipients relocating to an endemic area. Dig Dis Sci 2004;49: 1981-5. 86. Yorgin PD, Rewari M, al-Uzri AY, Theodorou AA, Scott KM, Barton LL. Coccidioidomycosis in adolescents with lupus nephritis. Pediatr Nephrol 2001;16:77-81. 87. Leake JA, Mosley DG, England B, Graham JV, Plikaytis BD, Ampel NM, et al. Risk factors for acute symptomatic coccidioidomycosis among elderly persons in Arizona, 1996-1997. J Infect Dis 2000;181:1435-40. 88. Deresinski SC, Stevens DA. Coccidioidomycosis in compromised hosts. Experience at Stanford University Hospital. Medicine 1975;54:377-95. 89. Johnson WM, Gall EP. Fatal coccidioidomycosis in collagen vascular diseases. J Rheumatol 1983;10:79-84. 90. Rutala PJ, Smith JW. Coccidioidomycosis in potentially compromised hosts: the effect of immunosuppressive therapy in dissemination. Am J Med Sci 1978;275:283-95. 91. Kauffman CA. Endemic mycoses in patients with hematologic malignancies. Semin Respir Infect 2002;17:106-12. 92. Blair JE, Smilack JD, Caples SM. Coccidioidomycosis in patients with hematologic malignancies. Arch Int Med 2005; 165:113-7. 93. Arsura EL, Kilgore WB, Ratnayake SN. Erythema nodosum in pregnant patients with coccidioidomycosis. Clin Infect Dis 1998;27:1201-3. 94. Caldwell JW, Arsura EL, Kilgore WB, Garcia AL, Reddy V, Johnson RH. Coccidioidomycosis in pregnancy during an epidemic in California. Obstet Gynecol 2000;95:236-9. 95. Braverman IM. Protective effects of erythema nodosum in coccidioidomycosis. Lancet 1999;353:168. 96. Walker MP, Brody CZ, Resnik R. Reactivation of coccidioidomycosis in pregnancy. Obstet Gynecol 1992;79:815-7. 97. Peterson CM, Schuppert K, Kelly PC, Pappagianis D. Coccidioidomycosis and pregnancy. Obstet Gynecol Surv 1993;48: 149-56. 98. Pappagianis D. Serologic studies in coccidioidomycosis. Semin Respir Infect 2001;16:242-50. 99. Cox A, Smith C. Arrested pulmonary coccidioidal granuloma. Arch Pathol 1939;27:717-34. 100. Bouza E, Dreyer JS, Hewitt WL, Meyer RD. Coccidioidal meningitis. An analysis of thirty-one cases and review of the literature. Medicine 1981;60:139-72. 101. Bried JM, Galgiani JN. Coccidioides immitis infections in bones and joints. Clin Orthop 1986;211:235-43. 102. Holley K, Muldoon M, Tasker S. Coccidioides immitis osteomyelitis: a case series review. Orthopedics 2002;25: 827-31, 32. 103. Cardone JS, Vinson R, Anderson LL. Coccidioidomycosis: the other great imitator. Cutis 1995;56:33-6. 104. Huntington RW Jr. Coccidioidomycosis—a great imitator disease. Arch Pathol Lab Med 1986;110:182. 105. DiCaudo DJ, Connolly SM. Interstitial granulomatous dermatitis associated with pulmonary coccidioidomycosis. J Am Acad Dermatol 2001;45:840-5. 106. Drutz DJ, Catanzaro A. Coccidioidomycosis. Part II. Am Rev Respir Dis 1978;117:727-71. 107. Smith C. Epidemiology of acute coccidioidomycosis with erythema nodosum (‘‘San Joaquin’’ or ‘‘valley fever’’. Am J Public Health 1940;30:600-11. 108. Psychos DN, Voulgari PV, Skopouli FN, Drosos AA, Moutsopoulos HM. Erythema nodosum: the underlying conditions. Clin Rheumatol 2000;19:212-6. 109. Cribier B, Caille A, Heid E, Grosshans E. Erythema nodosum and associated diseases. A study of 129 cases. Int J Dermatol 1998;37:667-72. 110. Richardson HB Jr, Anderson JA, McKay BM. Acute pulmonary coccidioidomycosis in children. J Pediatr 1967;70: 376-82. 111. DiCaudo DJ, Yiannias JA, Laman SD, Warschaw KE. The exanthem of acute pulmonary coccidioidomycosis: clinical and histopathologic features of three cases and review of the literature. Arch Dermatol 2006;142:744-6. 112. Werner SB. Coccidioidomycosis misdiagnosed as contact dermatitis. Calif Med 1972;117:59-61. J AM ACAD DERMATOL DiCaudo 941 VOLUME 55, NUMBER 6 113. Harvey WC, Greendyke WH. Skin lesions in acute coccidioidomycosis. Am Fam Physician GP 1970;2:81-5. 114. Ramras DG, Walch HA, Murray JP, Davidson BH. An epidemic of coccidioidomycosis in the Pacific beach area of San Diego. Am Rev Respir Dis 1970;101:975-8. 115. Huff JC, Weston WL, Tonnesen MG. Erythema multiforme: a critical review of characteristics, diagnostic criteria, and causes. J Am Acad Dermatol 1983;8:763-75. 116. Moreno AJ, Weisman I, Kenney RL, Fort SL. Concurrence of erythema multiforme and erythema nodosum. Cutis 1983; 31:275-8. 117. Brice SL, Huff JC, Weston WL. Erythema multiforme minor in children. Pediatrician 1991;18:188-94. 118. Brice SL, Huff JC, Weston WL. Erythema multiforme. Curr Probl Dermatol 1990;Jan/Feb:2-25. 119. Sweet RD. An acute febrile neutrophilic dermatosis. Br J Dermatol 1964;76:349-56. 120. Sweet RD. An acute febrile neutrophilic dermatosise1978. Br J Dermatol 1979;100:93-9. 121. Cohen PR, Kurzrock R. Sweet’s syndrome revisited: a review of disease concepts. Int J Dermatol 2003;42:761-78. 122. Burrall B. Sweet’s syndrome (acute febrile neutrophilic dermatosis). Dermatol Online J 1999;5(1):8. 123. Su WP, Fett DL, Gibson LE, Pittelkow MR. Sweet syndrome: acute febrile neutrophilic dermatosis. Semin Dermatol 1995; 14:173-8. 124. Fett DL, Gibson LE, Su WP. Sweet’s syndrome: systemic signs and symptoms and associated disorders. Mayo Clin Proc 1995;70:234-40. 125. von den Driesch P. Sweet’s syndrome (acute febrile neutrophilic dermatosis). J Am Acad Dermatol 1994;31:535-56. 126. Holemans X, Levecque P, Despontin K, Maton JP. Premiere association d’une coccidioidomycose et d’un syndrome de Sweet. Presse Med 2000;29:1282-4. 127. DiCaudo DJ, Ortiz KJ, Mengden SJ, Lim KK. Sweet syndrome (acute febrile neutrophilic dermatosis) associated with pulmonary coccidioidomycosis. Arch Dermatol 2005;141: 881-4. 128. Magro CM, Crowson AN, Regauer S. Granuloma annulare and necrobiosis lipoidica tissue reactions as a manifestation of systemic disease. Hum Pathol 1996;27:50-6. 129. Hobbs ER. Coccidioidomycosis. Dermatol Clin 1989;7:227-39. 130. Schwartz RA, Lamberts RJ. Isolated nodular cutaneous coccidioidomycosis. The initial manifestation of disseminated disease. J Am Acad Dermatol 1981;4:38-46. 131. Quimby SR, Connolly SM, Winkelmann RK, Smilack JD. Clinicopathologic spectrum of specific cutaneous lesions of disseminated coccidioidomycosis. J Am Acad Dermatol 1992; 26:79-85. 132. Hobbs ER, Hempstead RW. Cutaneous coccidioidomycosis simulating lepromatous leprosy. Int J Dermatol 1984;23:334-6. 133. Arsura EL, Kilgore WB, Caldwell JW, Freeman JC, Einstein HE, Johnson RH. Association between facial cutaneous coccidioidomycosis and meningitis. West J Med 1998;169:13-6. 134. O’Brien JJ, Gilsdorf JR. Primary cutaneous coccidioidomycosis in childhood. Pediatr Infect Dis 1986;5:485-6. 135. Winn WA. Primary cutaneous coccidioidomycosis. Reevaluation of its potentiality based on study of three new cases. Arch Dermatol 1965;92:221-8. 136. Levan NE, Huntington RW Jr. Primary cutaneous coccidioidomycosis in agricultural workers. Arch Dermatol 1965;92: 215-20. 137. Carroll GF, Haley LD, Brown JM. Primary cutaneous coccidioidomycosis: a review of the literature and a report of a new case. Arch Dermatol 1977;113:933-6. 138. Bonifaz A, Saul A, Galindo J, Andrade R. Primary cutaneous coccidioidomycosis treated with itraconazole. Int J Dermatol 1994;33:720-2. 139. Trimble J, Doucette J. Primary cutaneous coccidioidomycosis. Arch Dermatol 1956;74:405. 140. Wilson J, Smith C, Plunkett O. Primary cutaneous coccidioidomycosis: the criteria for diagnosis and report of a case. Calif Med 1953;79:233-9. 141. Chang A, Tung RC, McGillis TS, Bergfeld WF, Taylor JS. Primary cutaneous coccidioidomycosis. J Am Acad Dermatol 2003;49:944-9. 142. Overholt EL, Hornick RB. Primary cutaneous coccidioidomycosis. Arch Int Med 1964;114:149-53. 143. Pappagianis D. The phenomenon of locus minoris resistentiae in coccidioidomycosis. In: Einstein HE, Catanzaro A, editors. Coccidioidomycosis. Washington, DC: National Foundation for Infectious Diseases; 1985. pp. 319-29. 144. Zartarian M, Peterson EM, de la Maza LM. Detection of antibodies to Coccidioides immitis by enzyme immunoassay. Am J Clin Pathol 1997;107:148-53. 145. Kaufman L, Sekhon AS, Moledina N, Jalbert M, Pappagianis D. Comparative evaluation of commercial Premier EIA and microimmunodiffusion and complement fixation tests for Coccidioides immitis antibodies. J Clin Microbiol 1995;33:618-9. 146. Galgiani J. Coccidioides immitis. In: Mandell GL, Bennett JE, Dolin R, editors. Principles and practice of infectious diseases. Vol 2. Philadelphia: Churchill Livingstone; 2000. pp. 2746-57. 147. Huppert M, Sun SH, Rice EH. Specificity of exoantigens for identifying cultures of Coccidioides immitis. J Clin Microbiol 1978;8:346-8. 148. Padhye AA, Smith G, Standard PG, McLaughlin D, Kaufman L. Comparative evaluation of chemiluminescent DNA probe assays and exoantigen tests for rapid identification of Blastomyces dermatitidis and Coccidioides immitis. J Clin Microbiol 1994;32:867-70. 149. Sandhu GS, Kline BC, Stockman L, Roberts GD. Molecular probes for diagnosis of fungal infections. J Clin Microbiol 1995;33:2913-9. 150. Hayden RT, Qian X, Roberts GD, Lloyd RV. In situ hybridization for the identification of yeastlike organisms in tissue section. Diagn Mol Pathol 2001;10:15-23. 151. Bialek R, Kern J, Herrmann T, Tijerina R, Cecenas L, Reischl U, et al. PCR assays for identification of Coccidioides posadasii based on the nucleotide sequence of the antigen 2/prolinerich antigen. J Clin Microbiol 2004;42:778-83. 152. Greendyke WH, Resnick DL, Harvey WC. The varied roentgen manifestations of primary coccidioidomycosis. Am J Roentgenol Radium Ther Nucl Med 1970;109:491-9. 153. Batra P. Pulmonary coccidioidomycosis. J Thorac Imaging 1992;7:29-38. 154. Batra P, Batra RS. Thoracic coccidioidomycosis. Semin Roentgenol 1996;31:28-44. 155. McGahan JP, Graves DS, Palmer PE, Stadalnik RC, Dublin AB. Classic and contemporary imaging of coccidioidomycosis. Am J Roentgenol 1981;136:393-404. 156. Shetter AG, Fischer DW, Flom RA. Computed tomography in cases of coccidioidal meningitis, with clinical correlation. West J Med 1985;142:782-6. 157. Kim KI, Leung AN, Flint JD, Muller NL. Chronic pulmonary coccidioidomycosis: computed tomographic and pathologic findings in 18 patients. Can Assoc Radiol J 1998;49: 401-7. 158. Cohen AJ, Braunstein P, Pais MJ. The role of gallium and bone scintigraphy in disseminated coccidioidomycosis. Clin Nucl Med 1984;9:538-9. 942 DiCaudo J AM ACAD DERMATOL DECEMBER 2006 159. Zeppa MA, Laorr A, Greenspan A, McGahan JP, Steinbach LS. Skeletal coccidioidomycosis: imaging findings in 19 patients. Skeletal Radiol 1996;25:337-43. 160. Galgiani JN, Ampel NM, Catanzaro A, Johnson RH, Stevens DA, Williams PL. Practice guideline for the treatment of coccidioidomycosis. Infectious Diseases Society of America. Clin Infect Dis 2000;30:658-61. 161. Deresinski SC. Coccidioidomycosis: efficacy of new agents and future prospects. Curr Opin Infect Dis 2001;14:693-6. 162. Galgiani JN, Ampel NM, Blair JE, Catanzaro A, Johnson RH, Stevens DA, et al. Coccidioidomycosis. Clin Infect Dis 2005; 41:1217-23. 163. Ampel NM. Coccidioidomycosis in persons infected with HIV type 1. Clin Infect Dis 2005;41:1174-8. 164. Blair JE, Douglas DD, Mulligan DC. Early results of targeted prophylaxis for coccidioidomycosis in patients undergoing orthotopic liver transplantation within an endemic area. Transpl Infect Dis 2003;5:3-8. 165. Kappe R. Antifungal activity of the new azole UK-109, 496 (voriconazole). Mycoses 1999;42:83-6. 166. Li RK, Ciblak MA, Nordoff N, Pasarell L, Warnock DW, McGinnis MR. In vitro activities of voriconazole, itraconazole, and amphotericin B against Blastomyces dermatitidis, Coccidioides immitis, and Histoplasma capsulatum. Antimicrob Agents Chemother 2000;44:1734-6. 167. Gonzalez GM, Tijerina R, Najvar LK, Bocanegra R, Rinaldi M, Loebenberg D, et al. In vitro and in vivo activities of posaconazole against Coccidioides immitis. Antimicrob Agents Chemother 2002;46:1352-6. 168. Gonzalez GM, Tijerina R, Sutton DA, Graybill JR, Rinaldi MG. In vitro activities of free and lipid formulations of amphotericin B and nystatin against clinical isolates of Coccidioides immitis at various saprobic stages. Antimicrob Agents Chemother 2002;46:1583-5. 169. Hsue G, Napier JT, Prince RA, Chi J, Hospenthal DR. Treatment of meningeal coccidioidomycosis with caspofungin. J Antimicrob Chemother 2004;54:292-4. 170. Proia LA, Tenorio AR. Successful use of voriconazole for treatment of Coccidioides meningitis. Antimicrob Agents Chemother 2004;48:2341. 171. Clemons KV, Stevens DA. Efficacies of sordarin derivatives GM193663, GM211676, and GM237354 in a murine model of systemic coccidioidomycosis. Antimicrob Agents Chemother 2000;44:1874-7. 172. Antony S, Dominguez DC, Sotelo E. Use of liposomal amphotericin B in the treatment of disseminated coccidioidomycosis. J Natl Med Assoc 2003;95:982-5. 173. Koehler AP, Cheng AF, Chu KC, Chan CH, Ho AS, Lyon DJ. Successful treatment of disseminated coccidioidomycosis with amphotericin B lipid complex. J Infect 1998;36:113-5. 174. Pappagianis D. Seeking a vaccine against Coccidioides immitis and serologic studies: expectations and realities. Fungal Genet Biol 2001;32:1-9. 175. Delgado N, Xue J, Yu JJ, Hung CY, Cole GT. A recombinant beta-1,3-glucanosyltransferase homolog of Coccidioides posadasii protects mice against coccidioidomycosis. Infect Immun 2003;71:3010-9. 176. Cole GT, Xue JM, Okeke CN, Tarcha EJ, Basrur V, Schaller RA, et al. A vaccine against coccidioidomycosis is justified and attainable. Med Mycol 2004;42:189-216. 177. Jiang C, Magee DM, Quitugua TN, Cox RA. Genetic vaccination against Coccidioides immitis: comparison of vaccine efficacy of recombinant antigen 2 and antigen 2 cDNA. Infect Immun 1999;67:630-5. 178. Jiang C, Magee DM, Ivey FD, Cox RA. Role of signal sequence in vaccine-induced protection against experimental coccidioidomycosis. Infect Immun 2002;70:3539-45. 179. Zimmermann CR, Johnson SM, Martens GW, White AG, Zimmer BL, Pappagianis D. Protection against lethal murine coccidioidomycosis by a soluble vaccine from spherules. Infect Immun 1998;66:2342-5. 180. Kirkland TN, Raz E, Datta SK. Molecular and cellular mechanisms of protective immunity to coccidioidomycosis. Vaccine 2006;24:495-500.