* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File 9.08.16 the periodic table
Survey
Document related concepts
Transcript
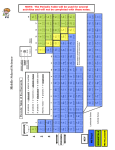
TALLOIDS NMETALS METALS KEY ERIODS 3 2 1 GROUP S Atomic mass -- # protons = # neutrons # protons = # electrons in a stable atom # protone + # neutrons = atomic mass # protons = element = atomic number NOTE: The Periodic Table will be used for several activities and will not be completed with these notes. TEKS 8.5C interpret the arrangement of the Periodic Table, including groups and periods, to explain how properties are used to classify elements TEKS 8.5B identify that protons determine an element’s identity and valence electrons determine its chemical properties, including reactivity The Periodic Table The modern periodic table was created by the Russian chemist, ____________ ____________. ______________ are represented on the Periodic Table with _____________ _______________. The elements are arranged from left to right based on their ___________ _______________. The atomic number comes from the number of _____________ present in stable atoms of that element. _____________ determine the element’s _______________! Here is the structure of the Periodic Table: Columns The ______________ on the Table are called ____________. The groups are numbered from _______________. Elements within groups have very similar ____________. On your Periodic Table, find this symbol: GROUPS Next to the symbol, draw arrows to show which direction the columns go. Which way do the groups go? _______________________________ Periods The _________ on the Periodic Table have meaning as well. The rows are called ____________. Next to the periods, draw this arrow to show the direction of the periods. Across any period, the _____________ of elements gradually change. For example, as you move from left to right across the table, atoms tend to become smaller in size, but ___________ in mass. Which way do the periods go?____________________________________ Key Notice the ______ at the top of the Periodic Table. This is a __________ that tells us what all of the ____________ and _____________ mean. Label the key on the Periodic Table. 14 Atomic _____________ Si Name = chemical symbol = ______________ symbol Atomic _____________ 28.068 Silicon Major Groups Elements are classified into ____________ large groups on the Periodic Table. The groups are _____________, _________________ and ___________________. Color the three groups on your periodic table and make a key. Use the colors listed below: Metals = blue Non-metals = green Metalloids = yellow KEY RIODS ___ ___ ___ GROUP S Atomic mass - # protons = # ________________ # protons = # _______________ in a stable atom # protons + # neutrons = __________ ________ # protons = _______________ = atomic number ___________ ___________ ___________ _____________