* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dobutamine stress echo-induced apical ballooning (Takotsubo
Heart failure wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Mitral insufficiency wikipedia , lookup
DiGeorge syndrome wikipedia , lookup
History of invasive and interventional cardiology wikipedia , lookup
Marfan syndrome wikipedia , lookup
Turner syndrome wikipedia , lookup
Electrocardiography wikipedia , lookup
Down syndrome wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Jatene procedure wikipedia , lookup
Echocardiography wikipedia , lookup
Coronary artery disease wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
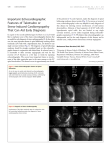
European Journal of Echocardiography (2009) 10, 395–399 doi:10.1093/ejechocard/jen292 Dobutamine stress echo-induced apical ballooning (Takotsubo) syndrome Ronan Margey*, Pauline Diamond, Hugh McCann, and Declan Sugrue Department of Cardiology, Mater Misericordiae University Hospital, Eccles Street, Dublin 7, Ireland Received 10 July 2008; accepted after revision 28 September 2008; online publish-ahead-of-print 22 October 2008 KEYWORDS Dobutamine stress echo; Apical ballooning; Takotsubo cardiomyopathy; Broken heart syndrome; Catecholamine-induced transient cardiomyopathy Introduction Takotsubo cardiomyopathy, also known as transient LV apical ballooning syndrome, was originally described in Japan in 1991, due to the resemblance of the LV ventriculogram to the appearance of a particular octopus pot.1,2 It has since been described in a number of ethnic groups.3,4 In a systematic review of this condition, it is estimated to account for 2% of all acute myocardial infarction presentations.4 It is most commonly triggered by significant emotional, physical, or mental stress, accounting for 30– 50% of all cases, although it has been described to occur with underlying medical disorders such as phaeochromocytoma, subarachnoid haemorrhage, exacerbation of bronchial asthma, Guillain-Barré syndrome, non-cardiac surgery, sepsis, and critical illness experienced by patients in intensive care units.5,6 Dobutamine stress echocardiography (DSE) is a commonly performed diagnostic non-invasive test to assess the stress-induced regional wall abnormalities indicative of ischaemia, and also to assess viability and contractile * Corresponding author. Tel: þ353 1 8034367; fax: þ353 1 8034775. E-mail address: [email protected] reserve in specific situations. Three case reports exist demonstrating the potential of DSE to induce apical ballooning syndrome, although the exact mechanism remains poorly understood. Herein, we describe a case of Takotsubo cardiomyopathy caused by DSE, and postulate that it occurred as a result of apical hyper-responsiveness to adrenergic stimulation. Case A 61-year-old lady was referred for assessment of exertional shortness of breath. She had a prior history of hypertension and a 40-pack year smoking habit. There was a family history of premature vascular disease. Her baseline electrocardiogram (ECG) showed hypertensive change, with inferolateral repolarization abnormalities, and on that basis, a dobutamine stress echocardiogram was performed (Figures 1–4). A standard dobutamine/atropine protocol was used with 10 mcg/kg/min dose increments at 3 min intervals. Her resting echocardiogram and blood pressure were normal. At 70% of her age-predicted heart rate, on 40 mg/min infusion of dobutamine, she developed typical cardiac chest Published on behalf of the European Society of Cardiology. All rights reserved. & The Author 2008. For permissions please email: [email protected]. Downloaded from by guest on October 14, 2016 Aims We report a case of dobutamine stress echocardiography (DSE) resulting in transient apical ballooning syndrome to highlight this rare condition as a potential complication of DSE. Background Takotsubo cardiomyopathy, or transient apical ballooning syndrome, is a recently described form of left ventricular (LV) dysfunction induced by stress. Clinically it can mimic acute coronary syndrome in its presentation. It is characterized by an atypical distribution of LV dysynergy with apical ballooning and compensatory basal hyperkinesis. Coronary angiography is normal. It has preponderance in females. Although the aetiology of Takotsubo syndrome remains obscure catecholamine release appears to be the principal trigger. Results We report a case of dobutamine-induced transient LV apical ballooning in a woman without coronary disease, during a dobutamine stress echocardiogram. There was evidence of ventricular recovery by 72 h. To our knowledge, only three other case reports describe dobutamine-induced Takotsubo cardiomyopathy. Conclusion Dobutamine stress echocardiography is a widely performed diagnostic test, however, it can rarely result in presumed catecholamine-induced transient apical ballooning syndrome. 396 R. Margey et al. Figure 3 Baseline apical four-chamber view at end-systole. Figure 2 Baseline short-axis view at end-systole. Figure 4 Baseline apical two-chamber view at end-systole. Downloaded from by guest on October 14, 2016 Figure 1 Baseline parasternal long-axis view at end-systole. pain, with associated inferolateral ST elevation, hyperacute anterolateral T-waves, and ventricular bigeminy. On review of the DSE images, it was apparent that there was severe akinesis of the apical, anteroseptal, and apicolateral segments at peak dobutamine infusion (Figures 5 and 6). No evidence of a mid-cavity obstruction gradient was demonstrated. She was immediately transferred to the cardiac catheterization laboratory and underwent coronary angiography. Her epicardial vessels were normal and a mid-left anterior descending coronary artery segment of bridging was noted. Left ventriculography revealed hyperdynamic basal myocardial segments with near-cavity obliteration, distal anterior, inferior, lateral, and apical akinesis, and the LV end diastolic pressure was 22 mmHg (Figures 7–9). She was transferred to the coronary care unit, where she was commenced on beta-blockade, and anti-coagulated. Troponin I peaked at 4.8 ng/dL (0.01–0.04) and creatinine kinase peaked at 243 mg/dL (21–232). Her in-hospital course was complicated by atrial fibrillation with rapid ventricular response rates, requiring chemical cardioversion with amiodarone, and by mild left ventricular (LV) failure, requiring intravenous diuresis. Repeat echocardiography at 72 h showed near-normal LV function with mild residual apical and anteroseptal hypokinesia. Figure 5 Apical four-chamber view at peak dobutamine infusion, end-systole [note the large apical balloon (arrow)]. Repeat cardiac catheterization before discharge on day five, revealed markedly improved LV function, with an estimated ejection fraction of 50–55%, and mild residual anteroapical hypokinesia. Apical ballooning syndrome 397 Figure 6 Apical two-chamber view at peak dobutamine infusion at end-systole [note the apical ballooning (arrow)]. Figure 8 Left coronary angiogram anteroposterior projection cranial view. Downloaded from by guest on October 14, 2016 Figure 7 Left ventriculogram at end-systole right anterior oblique projection view (note the apical ballooning). She has been symptom free since discharge and repeat echocardiogram at 8 weeks, revealed complete recovery of LV function. Figure 9 Right coronary angiography left anterior oblique projection cranial view. Discussion Takotsubo cardiomyopathy is a recently described clinical condition, originally described in 1991.1 Typically, it presents with symptoms and signs resembling an acute coronary syndrome, which can lead to inappropriate therapy, e.g. thrombolytic administration.7,8 It has preponderance in females (9:1). In most reported series, it commonly affects post-menopausal women, with an average age range of 62–75 years, although it has been reported in individuals aged 10–91 years.8 It commonly presents with chest pain (68%), although it may present with shortness of breath (20%), cardiogenic shock (4%), or ventricular arrhythmia (2%).5–7 Electrocardiogram typically shows ST elevation typical of acute myocardial infarction, deep global T-wave inversion, or prolongation of the QT interval; and the ECG changes can affect multiple territories. The ECG changes typically resolve over within months.7,8 It is associated with mild elevation in cardiac enzymes, disproportionately low given the extent of wall motion abnormality.7,8 Echocardiography shows a typical appearance of significant LV dysfunction, with preserved basal segment function, and moderate to severe dysfunction of the mid- and apical segments. The echocardiographic appearance improves rapidly over 3–5 days. 398 this, it is well described that females appear more vulnerable to sympathetically mediated stunning, and postmenopausal alteration of endothelial function in response to decreased oestrogen levels has been associated advocated as a possible explanation.11 A recent paper postulated that the condition arises due to the hyperdynamic basal segments creating an intracavity gradient causing excess release or dehydration of catecholamine, resulting in an isolated apical chamber that produces myocardial stunning without infarction.12–14 It is known that up to 20% of patients undergoing DSE develop a dynamic LV mid-cavity obstruction, and perhaps this reflects the potential mechanism of apical ballooning induced by DSE.13,14 In our case, no clear emotional or stress trigger could be identified. The only apparent initiation factor would appear to be dobutamine infusion. We postulate that increased apical responsiveness to adrenergic stimulation previously described, offers a potential mechanism as to how DSE could culminate in transient apical ballooning. In addition to overstimulation of the apical adrenergic receptors, dobutamine may also has worsened the hyperdynamic basal systolic function, creating an artificial LVOT gradient, and further stressing the myocardium, which was not demonstrated in our case. This mechanism, however, was demonstrated in a previous series where patients with Takotsubo cardiomyopathy underwent low-dose DSE following recovery, which provoked an LV mid-cavity gradient at peak dose.14 Conclusion Dobutamine stress echocardiography is a widely performed test, and is largely safe, although induction of myocardial infarction is well recognized. Our report highlights the potential of DSE to induce transient apical ballooning, through a combination of adrenergic overstimulation and LV mid-cavity obstruction. All centres performing DSE should be aware of the potential complication of apical ballooning syndrome. Conflict of interest: none declared. Funding R.M. research position is kindly funded by an educational grant from the Irish Heart Foundation, the Health Services Executive of Ireland, and by an unrestricted educational bursary from Medtronic Corporation, Ireland. References 1. Dote K, Sato H, Tateishi H, Uchida T, Ishihara M. Myocardial Stunning due to simultaneous multivessel coronary spasms: a review of 5 cases. J Cardiol 1991;21:203–14. 2. Abe Y, Kondo M, Matsuoka R, Araki M, Dohyama K, Tanio H. Assessment of clinical features in transient left ventricular apical ballooning. J Am Coll Cardiol 2003;41:737–42. 3. Kawai S, Suzuki H, Yamaguchi H, Tanaka K, Sawada H, Aizawa T et al. Ampulla cardiomyopathy (‘Takotsubo’ cardiomyopathy)-reversible left ventricular dysfunction: with ST segment elevation. Jpn Circ J 2000;64: 156–9. 4. Desmet WJ, Adriaenssens BF, Dens JA. Apical Ballooning of the left ventricule: first series in white patients. Heart 2003;89:1027–31. Downloaded from by guest on October 14, 2016 Cardiac magnetic resonance (CMR) does not show any evidence of myocardial necrosis, and endomyocardial biopsy tends to show a mononuclear infiltrate without any evidence of myocarditis or myocardial necrosis. Occasionally, contraction band necrosis can be observed, which is well described in catecholamine-induced myocyte injury.5,7,8 Angiography in the vast majority of described cases shows normal coronaries. Spontaneous or provoked multivessel epicardial vessel spasm has been described.5 The combination of apical and mid-ventricular wall motion abnormalities can cause intracavity LV gradient, which can cause haemodynamic instability, and result in systolic anterior motion of the anterior mitral leaflet, producing posteriorly directed mitral regurgitation.8 Recovery is usually rapid, although heart failure, cardiogenic shock, ventricular arrhythmia, mitral incompetence, LV outflow tract (LVOT) obstruction, and free wall rupture have all been described as a complication of this condition. In the published literature, it is associated with a 3.5% risk of recurrence. Right ventricular dysfunction has been described in up to one-third of cases. These patients are particularly prone to LV thrombus formation.7,8 In-hospital mortality has been estimated at 1.1%, with up to 20% experiencing heart failure. The commonest reported causes of mortality are cardiogenic shock and thromboembolism.8 Management is largely supportive, with fluid resuscitation (if no pulmonary congestion), beta-blockade, and occasionally afterload augmentation with phenylephrine in those with an LVOT gradient. Consideration should be given to therapeutic anti-coagulation to prevent thromboembolism. For those with haemodynamic instability, inotropes and intra-aortic balloon counterpulsation may be required.7,8 The exact mechanism of occurrence remains poorly understood, but several hypotheses exist. Epicardial coronary spasm has been demonstrated in up to 11% of reported cases. Provoked spasm has been demonstrated in the catheterization laboratory, but its relevance remains unclear. Microvascular spasm and microvascular obstruction have been hypothesized, but the lack of subendocardial infarction on CMR undermines this theory.5,8 Catecholamine levels are significantly elevated in individuals with this condition, reflecting increased synthesis, reuptake, and removal metabolism of the adrenergic hormones.5 This may lead to catecholamine-induced cyclic adenylate monophosphate calcium overload of the myocyte, resulting in direct myocyte injury.5 Catecholamines may also stimulate oxygen-free radical generation, which can cause local myocyte injury.5 Interestingly, it has been reported that the apical myocardium has an increased response to adrenergic stimulation, and may be vulnerable to surges in circulating catecholamine levels.9 Local release of catecholamines from adrenergic neurones in the myocardium seems unlikely as there is a higher norepinephrine content and concentration of sympathetic nerves at the base of the heart compared with the apex.9 A base-to-apex perfusion gradient may exist as occurs in individuals with coronary risk factors.10 Finally, sex differences may account for the condition occurring predominately in females, although it is worthwhile noting that higher circulating basal levels of catecholamines occur in men, and males produce a more marked elevation in catecholamines in response to stress.6 Despite R. Margey et al. Apical ballooning syndrome 5. Gianni M, Dentali F, Grandi A, Sumner G, Hiralal R, Lonn E. Apical ballooning syndrome or Takotsubo cardiomyopathy: a systematic review. Eur Heart J 2006;27:1523–9. 6. Wittstein I, Thiemann D, Lima J, Baughman K, Schulman S, Gerstenblith G et al. Neurohumoral features of myocardial stunning due to sudden emotional stress. N Engl J Med 2005;352:539–48. 7. Bybee KA, Kara T, Prasad A, Lerman A, Barsness GW, Wright RS et al. Systematic review: transient left ventricular apical ballooning: a syndrome that mimics ST-segment elevation myocardial infarction. Ann Intern Med 2004;141:858–65. 8. Bybee KA, Prasad A. Stress-related cardiomyopathy syndromes. Circulation 2008;118:397–409. 9. Mori H, Ishikawa S, Kojima S, Hayashi J, Watanabe Y, Hoffman J et al. Increased responsiveness of left ventricular apical myocardium to adrenergic stimuli. Cardiovasc Res 1993;27:192–8. 399 10. Hernandez-Pampaloni M, Keng FY, Kudo T, Sayre J, Schelbert HR. Abnormal longitudinal, base-to-apex myocardial perfusion gradient by quantitative blood flow measurements in patients with coronary risk factors. Circulation 2001;31:527–32. 11. Taddei S, Virdis A, Ghiadani L, Mattei P, Sudano I, Berini G et al. Menopause is associated with endothelial dysfunction in women. Hypertension 1996;28:576–82. 12. Silberbauer J, Hong P, Lloyd GW. Takotsubo cardiomyopathy (left ventricular ballooning syndrome) induced during dobutamine stress echocardiography. Eur J Echocardiogr 2008;9:136–8. 13. Cherian J, Kothari S, Angelis D, Downey B, Kirkpatrick J Jr. Atypical Takotsubo cardiomyopathy: dobutamine-precipitated apical ballooning with left ventricular outflow tract obstruction. Tex Heart Inst J 2008;35:73–5. 14. Merli E, Sutcliffe S, Gori M, Sutherland G. Takotsubo cardiomyopathy: new insights into the possible underlying pathophysiology. Eur J Echo 2006;7:53–61. Downloaded from by guest on October 14, 2016