* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Case report Homozygous Tangier disease with undetectable serum
Frameshift mutation wikipedia , lookup
Nutriepigenomics wikipedia , lookup
Designer baby wikipedia , lookup
Medical genetics wikipedia , lookup
Genome (book) wikipedia , lookup
Tay–Sachs disease wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Fetal origins hypothesis wikipedia , lookup
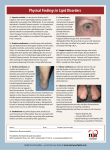
Page 1 of 3 Clinical Genetics Case report Homozygous Tangier disease with undetectable serum high density lipoprotein cholesterol levels and no clinical features Abstract Introduction Tangier disease is a very rare inherited disorder characterised by much reduced high-density lipoprotein levels, large yellow-orange tonsils and enlarged liver, spleen and lymph nodes. It is caused by mutations in the ATP-binding cassette transporter A1 gene. This paper reports a case of homozygous Tangier disease with undetectable serum high density lipoprotein cholesterol levels and no clinical features. Case report We report a study of a 40-year-old female who presented with undetectable high-density lipoprotein cholesterol but no clinical signs of Tangier disease. Her family history is significant for her father having premature cardiovascular disease and a moderately low high-density lipoprotein cholesterol and other relatives having low or undetectable highdensity lipoprotein cholesterol levels. She was found to have a homozygous mutation in the ATP-binding cassette transporter A1 gene. Conclusion As Tangier disease may not have clinical features, it may be underdiagnosed, and as more patients are reported in the literature, the variation in phenotype will become apparent. Introduction Tangier disease is one of the most severe forms of familial high-density lipoprotein (HDL) deficiency. Since its discovery on Tangier Island in *Corresponding Author: C van Heyningen Email: [email protected] Clinical Laboratories, Aintree University Hospital, Lower Lane, Liverpool L9 7AL, UK Chesapeake Bay (Virginia, USA) in 19611, Tangier disease has been diagnosed in about 100 patients worldwide2. It is characterised by severe deficiency or absence of apoA-I, the major HDL apolipoprotein, and in some cases by the accumulation of cholesteryl esters in tissues throughout the body including tonsils, peripheral nerves, intestinal mucosa, spleen, liver, bone marrow, lymph nodes, thymus, skin and cornea. The biochemical signs of this condition include serum HDL cholesterol concentration less than 0.12 mmol/L, apoA-I levels below 0.05 g/L, low total serum cholesterol below 3.9 mmol/L and normal or high serum triglycerides. The major clinical signs of Tangier disease are hyper-plastic orange-yellow tonsils, hepatosplenomegaly, neuropathy, corneal opacities, thrombocytopenia, anaemia and stomatocytosis. These clinical signs combine differently in each patient2 and we report a patient without physical signs identified by finding undetectable serum levels of HDL cholesterol. Case report A 40-year-old female was referred to the lipid clinic because of persistent undetectable serum HDL cholesterol (HDL-C) levels of <0.1 mmol/L. The other serum lipids had varied with total cholesterols of 2.9 to 2.3 mmol/L and triglycerides of 2.5 to 1.7 mmol/L. Serum apolipoprotein A1 was found to be undetectable at < 0.05 g/L (reference range: 1.25–2.15) and apolipoprotein B was within the normal adult reference range of 0.55 to 1.25 g/L at 0.95 g/L. Fasting plasma glucose was 4.7 mmol/L and renal, liver, thyroid function tests and a blood cell count were normal. On microscopy a blood film showed anisopoikilocytosis with acanthocytes and elliptocytes. A very low serum HDL-C was first recorded during her first pregnancy at age 23 years. At age 35 years, she underwent a bilateral oophorectomy and hysterectomy for endometriosis. She takes oestradiol 1 mg/day as hormone replacement therapy and tolterodine 4 mg/day, an antimuscarinic for urinary incontinence, and no over-the-counter medication or supplements. She stopped smoking 10 years ago, keeps to a healthy diet, takes exercise and only occasionally drinks alcohol. Physical examination showed no discolouration of tonsils or pharynx, no enlargement of liver, spleen or lymph nodes, no peripheral neuropathy and no corneal opacities. She had a blood pressure of 113/67 mmHg and body mass index of 30 kg/square metre. Computed tomography studies showed mild calcification of the right coronary artery but no stenosis was found on angiography. She was found to have family members with reduced HDL-C levels. Her brother aged 37 years has schizophrenia and a serum HDL-C <0.1 mmol/L. Her father, her 17-year-old daughter and 11-year-old son have low levels of HDL-C of 0.8 mmol/L, 1.0 mmol/L and 0.8 mmol/L, respectively. Her father presented at age 40 years with a stroke and ischaemic heart disease. Genetic analysis was undertaken in the medical genetics department, Oslo University Hospital, Norway. DNA sequencing of the translated exons with flanking intron sequences of the apolipoprotein A-I (apoA-I) and lecithin-cholesterol acyltransferase (LCAT) genes did not identify Licensee OA Publishing London 2013. Creative Commons Attribution Licence (CC-BY) F : Gell J, van Heyningen C. Homozygous Tangier disease with undetectable serum high density lipoprotein cholesterol levels and no clinical features. OA Case Reports 2013 Oct 21;2(13):126. CompeƟng interests: none declared. Conflict of interests: none declared. All authors contributed to the concept on, design, and preparaƟon of the manuscript, as well as read and approved the final manuscript. All authors abide by the AssociaƟon for Medical Ethics (AME) ethical rules of disclosure. J Gell, C van Heyningen* Page 2 of 3 Case report Discussion Tangier disease is caused by mutations in the ABCA1 gene which encodes the membrane transporter ABCA13. The ABCA1 gene resides on chromosome 9q22-q31, contains 50 exons, and codes for a 2261-amino acid long membrane protein4. In this case, the mutation was found to be a true homozygote for mutation in exon 14 in the ABCA1 gene. This is a duplication that causes a frame shift, leading to a premature stop codon being generated 43 codons downstream of the mutation, which in turn generates a non-functional protein. This transporter normally plays a key role in the first step of reverse cholesterol transport, through which the efflux of free cholesterol from non-hepatic peripheral tissues is transferred to HDL by the ATP-binding cassette transporter. Lipid-poor apoA-I acts as an acceptor, and the phospholipid component of HDL acts as a sink for the mobilised cholesterol and thereby plays a central role in both regulating cellular cholesterol homeostasis and forming HDL. A non-functional ABCA1 impairs free cholesterol efflux from cells. This may lead to intracellular accumulation of esterified cholesterol, prevention of lipid-poor apoA-I particles converting into pre-β HDL and rapid catabolism of the poorly lipidated apoA-I primarily by the kidney5. This rapid catabolism explains why this patient has very low levels of apoA-I despite a normal apoA-I gene. There has been an on-going debate as to whether HDL deficiency in Tangier disease is caused by increased catabolism or by the decreased synthesis of apolipoproteins. A number of studies using radiolabeled HDL, apoA-I and apoA-II in Tangier disease affected patients have been performed and reveal a markedly increased catabolism of apoA-I, apoA-II and HDL. These studies support the metabolic basis of Tangier disease being a rapid catabolism of apoA-I and HDL6. In our patient, a frame-shift mutation leads to the formation of non-functional ABCA1 proteins which in turn prevents the uptake of free cholesterol and leads to the loss of HDL particles. Epidemiological studies demonstrate an inverse association between low levels of HDL cholesterol and increased risk of ischaemic heart disease, but population studies suggest that genetically low HDL cholesterol per se does not predict an increased risk of ischaemic heart disease7. Reduced HDL is associated with cardiovascular disease by its function in regulating cholesterol efflux, modulation of the inflammatory response, antioxidant activity and vasomotor regulation. Some believe that the main protective effect of HDL in preventing cardiovascular disease is by inhibiting the oxidative modifications of low density lipoprotein8, an initial step in the atherosclerotic process. The literature on homozygous Tangier disease indicates a strong association with cardiovascular disease and premature onset of coronary artery disease9. To date, there is no specific treatment for Tangier disease. The hypothesis that elevation of HDL reduces the atherosclerotic burden and/or decreases ischaemic cardiovascular events in humans has unfortunately been impeded by a lack of drugs that selectively increase HDL. Most of the drugs designed to increase HDL levels, have not been shown to be effective in patients with Tangier disease2. Our patient has no clinical evidence of cardiovascular disease but imaging studies of her coronary arteries show mild calcification without stenosis. As her father has low HDL cholesterol and presented with premature cardiovascular disease, it is likely that she too has a high risk for cardiovascular events. Even though her total cholesterol is low, she may benefit in the long term from low density lipoprotein cholesterol lowering therapy as well as weight reduction and a very low fat diet. Conclusion This case study illustrates how a patient with Tangier disease without clinical features may be identified by serum lipid profile screening and finding an undetectable HDL-C level. The diagnosis must be confirmed by genetic analysis of genes associated with low HDL-C. The degree of risk for cardiovascular disease may be estimated by studying the family history of lipid abnormalities and cardiovascular events. Consent Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal. Acknowledgements We thank the Department of Medical Genetics, Oslo University Hospital, Norway for genetic analysis. References 1. Fredrickson DS, Altrocchi PH, Avioli LV, et al. Tangier disease. Combined clinical staff conference at the National Institute of Health. Ann Intern Med. 1961 Dec;55:1016. 2. Puntoni M, Sbrana F, Bigazzi F and Sampietro T. Tangier disease: epidemiology, pathophysiology, and management. Am J Cardiovasc Drugs. 2012 Oct;12(5):303–11. 3. Brunham LR, Singaraja RR, Hayden MR. Variation on a gene: rare and common Licensee OA Publishing London 2013. Creative Commons Attribution Licence (CC-BY) F : Gell J, van Heyningen C. Homozygous Tangier disease with undetectable serum high density lipoprotein cholesterol levels and no clinical features. OA Case Reports 2013 Oct 21;2(13):126. CompeƟng interests: none declared. Conflict of interests: none declared. All authors contributed to the concept on, design, and preparaƟon of the manuscript, as well as read and approved the final manuscript. All authors abide by the AssociaƟon for Medical Ethics (AME) ethical rules of disclosure. any mutation. DNA sequencing of the translated exons of the ATP-binding cassette transporter A1 (ABCA1) gene revealed that the patient is a true homozygote for mutation c.1758dupG in exon 14 in the ABCA1 gene. This mutation is a duplication that causes a frame shift, which leads to a premature stop codon being generated 43 codons downstream of the mutation (p.Arg587AlafsX43). This results in a non-functional protein and indicates a diagnosis of Tangier disease. Page 3 of 3 Case report 6. Schaefer EJ, Brousseau ME, Diffenderfer MR, Cohn JS, Welty FK, O’Connor J Jr. et al. Cholesterol and apolipoprotein B metabolism in Tangier disease. Atherosclerosis. 2001;159(1):231–36. 7. Frikke-Schmidt R. Genetic variation in the ABCA1 gene, HDL cholesterol, and risk of ischemic heart disease in the general population. Atherosclerosis. 2010 Feb;208(2):305–16. 8. Navab M, Imes SS, Hama SY, et al. Monocyte transmigration induced by modification of low density lipoprotein in co-cultures of human aortic wall cells is due to induction of monocyte chemotactic protein-1 synthesis and is abolished by high density lipoprotein. J Clin Invest. 1991 Dec;88:2039–46. 9. Serfaty-Lacrosniere C, Civeira F, Lanzberg A, Isaia P, Berg J, Janus ED et al. Homozygous Tangier disease and cardiovascular disease. Atherosclerosis. 1994 May;107(1):85–98. Licensee OA Publishing London 2013. Creative Commons Attribution Licence (CC-BY) F : Gell J, van Heyningen C. Homozygous Tangier disease with undetectable serum high density lipoprotein cholesterol levels and no clinical features. OA Case Reports 2013 Oct 21;2(13):126. CompeƟng interests: none declared. Conflict of interests: none declared. All authors contributed to the concept on, design, and preparaƟon of the manuscript, as well as read and approved the final manuscript. All authors abide by the AssociaƟon for Medical Ethics (AME) ethical rules of disclosure. variants in ABCA1 and their impact on HDL cholesterol levels and atherosclerosis. Annu Rev Nutr. 2006;26:105–29. 4. Von Eckardstein A. Differential diagnosis of familial high density lipoprotein deficiency syndromes. Atherosclerosis. 2006;186(2):231–39. 5. Oram JF, Heinecke JW. ATP-binding cassette transporter A1: a cell cholesterol exporter that protects against cardiovascular disease. Physiol Rev. 2005;85:1343–72.