* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Anesthetic Strategies for Triple Endoscopy
Survey
Document related concepts
Transcript
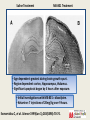
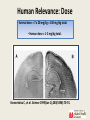
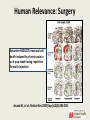
Anesthetic Strategies for Triple Endoscopy 4th Annual Contemporary Management of Aerodigestive Disease in Children Vanderbilt University Medical Center - Nashville, TN - 11/04/16 Stephen Robert Hays, MS, MD, FAAP Associate Professor, Anesthesiology & Pediatrics Vanderbilt University Medical Center Pediatric Anesthesia/Pediatric Pain Service/Pediatric Pain Clinic Monroe Carell Jr. Children’s Hospital at Vanderbilt CME Requirements: Objectives • Review recent animal data indicating significant adverse neuropathology and behavioral abnormalities following anesthetic exposure in developmentally vulnerable subjects. • Discuss retrospective data in humans describing associations between anesthetic exposure in infants and young children and subsequent developmental disability, as well as prospective data suggesting otherwise. • Consider relevance of available data to human anesthetic exposure, with particular consideration of anesthetic strategies for triple endoscopy in pediatric patients. CME Requirements: Disclosures I have no relevant financial relationships with any commercial interests. Vanderbilt is a site for GAS, PANDAS, HIP. Site investigator for industry-sponsored pediatric licensing studies including: IR/ER oxymorphone - Opana®, ENDO ER oxycodone - OxyContin®, Purdue Pharma IV acetaminophen - Orfimev®, Mallinckrodt ER hydromorphone - Exalgo®, Mallinckrodt Tapentadol - Nucynta®, Janssen (IV dexmedetomidine - Precedex®, Hospira) CME Requirements: Off-Label Many anesthetic agents and techniques are widely used in children; Many such agents and techniques are NOT approved for such use. Much of current pediatric anesthetic practice, including strategies for triple endoscopy, are still largely off-label/investigational. Neonatal Pain & Adverse Outcomes •Tobiansky R, et al. J Paediatr Child Health 1995(Jun);31(3):233-236. •Chacko J, et al. Pediatr Surg Int 1999;15(7):496-499. •American Academy of Pediatrics. Pediatrics 2000(Feb);105(2):454-461. •Oberlander TF, et al. Pediatrics 2000(Jan);105(1):e6. •Bhutta AT, Anand KJ. Clin Perinatol 2002(Sep);29(3):357-372. •Grunau R. Clin Perinatol 2002(Sep);29(3):373-394,vii-viii. •Fredriksson A, et al. Behav Brain Res 2004(Aug);153(2):367-376. •Anand KJ, et al. Pediatrics 2006(Mar);117:S9-S22. •Grunau RE, et al. Semin Fetal Neonatal Med 2006(Aug);11(4):268-275. •Whitfield MF, Grunau RE. Clin Perinatol 2000(Jun);27(2):363-379. Providing inadequate anesthesia is NOT appropriate. Provocative Urologic Example Taddio A, et al. Lancet 1995(Feb 4);345(8945):291-2. Male infants circumcised as neonates exhibited exaggerated pain behavior with subsequent immunization compared to uncircumcised male infants. Taddio A, et al. Lancet 1997(Mar 1);349(9052):599-603. Confirmed the above; also demonstrated blunting of exaggerated pain behavior with subsequent immunization if circumcision performed under topical anesthesia. Even seemingly minor procedures are of potential concern. Ikonomidou C, et al. Science 1999(Jan 1);283(5398):70-74. Developmental Neuroapoptosis Developing neurons are programmed to commit suicide if they are unsuccessful in meeting important developmental milestones: • Such pruning is a normal part of development. Many developing neurons successfully meet such milestones, so not all are obliged to commit suicide: • 1-2% of neurons at any given time are lost in this manner. • Up to 50% of neurons in some loci may be eliminated. Aberrant circumstances can interfere and cause: • Failure of many neurons to meet important milestones; • Death of many neurons that would have normally survived; • Abnormal neurodevelopmental disturbances. Saline Treatment MK-801 Treatment • Age dependent: greatest during brain growth spurt. • Region dependent: cortex, hippocampus, thalamus. • Significant apoptosis began by 4 hours after exposure. • Initial investigations with MK-801 = dizocilpine. • Ketamine: 7 injections of 20mg/kg over 9 hours. Ikonomidou C, et al. Science 1999(Jan 1);283(5398):70-74. Olney JW, et al. Anesthesiology 2004(Aug);101(2):273-275. Mellon RD, et al. Anesth Analg 2007(Mar);104(3):509-520. Human Relevance? Animal study design issues: • Dose differences: veterinary doses generally much higher. • Durations of exposure: animal exposure generally much longer. • Timing of exposure: animal development is not the same as human. • No painful stimulus: animal exposure not for surgery. • Inescapable uncertainty of inter-species comparisons. Human Relevance: Dose • Animal dose = 7 x 20 mg/kg = 140 mg/kg total. • Human dose = 1-2 mg/kg total. Ikonomidou C, et al. Science 1999(Jan 1);283(5398):70-74. Human Relevance: Duration Perinatal Development: 7 rat days 27 human months. 6 rat hours 1 human month. Soriano S. Presentation to FDA Advisory Committee, Spring 2007. Human Relevance: Timing Correlation of developmental stages in animals in variable, non-linear, and of obvious crucial importance: Human Relevance: Surgery Ketamine REDUCES neuronal cell death induced by chronic pain in a rat pup model using repetitive formalin injection. Anand KJ, et al. Pediatr Res 2007(Sep);62(3):283-290. Human Relevance: Inter-Species Human Infants Rat Pups! “Rodent data provide an imprecise basis at best, and an irrelevant basis at worst, for evaluating human risk. An important next step, therefore, would be to conduct well designed nonhuman primate studies.” Olney JW, et al. Anesthesiology 2004(Aug);101(2):273-275. Primate Anesthetic Exposure • Prolonged (24 h) ketamine exposure induces apoptotic neuronal cell death in perinatal monkey brain. • Shorter (3 h) ketamine exposure does NOT. • Developmental immaturity (GD 122, PND 5; NOT PND 35) represents a critical period of particular vulnerability. • Up-regulation of NMDA receptor (NR-1 mRNA) correlates with neuropathology. Slikker W, et al. Toxicol Sci 2007(Jul);98(1):145-58. Anesthetic and Life Support Drugs Advisory Committee March 2007 1. Please discuss whether there are sufficient data to determine the applicability of the findings for anesthetics in non-clinical models to humans? If not, what other data would be needed? 2. To what extent are the doses and durations of exposure to the anesthetics used in non-clinical studies relevant to the clinical use of these drugs? 3. Combinations of anesthetic drug products are frequently used in the setting of pediatric anesthesia. Most of the preclinical data are derived from studies of drugs examined in isolation. Does the Committee have any advice on how FDA may best approach the issue of neurologic toxicity of combination use? Anesthetic and Life Support Drugs Advisory Committee March 2007 4. Are there feasible clinical or other study designs to assess the potential neurological toxicities of exposing pediatric patients to anesthetic agents? (Please discuss) 5. Given the risks associated with delay of surgical intervention or with the use of sub-optimal anesthesia techniques, how does one incorporate the current knowledge base into the practice of pediatric anesthesia? “there are many issues to be resolved before a definitive assessment of the risk posed by anesthetics to the developing brain can be made.” “there are no clinical data providing evidence suggesting the use of anesthetics in the neonate or young child is associated with signs of developmental neurotoxicity.” Anesthetic and Life Support Drugs Advisory Committee March 2007 The committee unanimously voted no change in current practice is indicated, including no change in current drug labeling. The committee acknowledged relevance of animal data to anesthetic exposure in human infants is unknown. The committee agreed more specific patient research is indicated, particularly regarding long term follow-up. Anesthetic and Analgesic Drug Products Advisory Committee March 2011 The committee upheld previous recommendations: “…progress has not been substantial and there is still too little information to draw any firm conclusions.” 6-day old mice exposed to 3% sevoflurane for 6 h. Satomoto M, et al. Anesthesiology 2009(Mar);110(3):628-37. Satomoto M, et al. Anesthesiology 2009(Mar);110(3):628-37. Forgetful Mice Autistic Children Animal Data: Summary Parameter Neurotoxicity Comment Human Correlate Timing of exposure Only following exposure during Window varies by species ? 3rd trimester - ? age 3 y; critical neurodev window Anesthetic dose Only following exposure above Potentially much longer Dose varies by agent, species Unknown critical threshold dose Anesthetic duration Only following exposure above critical threshold duration Concomitant pain Duration varies by agent, Unknown species Only following exposure without Untreated pain is neurotoxic; Untreated pain neurotoxic; concomitant pain Analgesia is neuroprotective Analgesia neuroprotective Retrospective analysis of healthy children having urologic procedures before age 6 y. Kalkman CJ, et al. Anesthesiology 2009(Apr);110(4):805-12. Retrospective analysis of all children born in Olmsted County, MN between 1976 and 1982 who received general anesthesia before age 4. Wilder RT, et al. Anesthesiology 2009(Apr);110(4):796-804. Conclusion: Exposure to anesthesia was a significant risk factor for the later development of LD in children receiving multiple, but not single anesthetics. These data cannot reveal whether anesthesia itself may contribute to LD or whether the need for anesthesia is a marker for other unidentified factors that contribute to LD. Wilder RT, et al. Anesthesiology 2009(Apr);110(4):796-804. Retrospective Human Data: Summary Parameter Neurotoxicity Comment Age at exposure Birth through 10 years Multiple cohorts studied Single anesthetic exposure No consistent association with ↑ risk of deficit Multiple assessment parameters Multiple anesthetic exposures Consistent association with ↑ risk of deficit Multiple assessment parameters Anesthetic type/dose Causality unknown Generally not specified/quantified Prospective Human Studies Study (Scope) Design Assessments Comparison Enrolment (Contact) Human Subject Study Retrospective cohort; Hippocampal memory Anesthesia <8y v. Ongoing (goal 300 pts) (single institution) Prospective evaluation No anesthesia UCSF: Jeffrey Sall MASK Retrospective cohort; Neurocognitive Anesthesia <3y v. Ongoing (goal 500 pts) (single institution) Prospective evaluation battery No anesthesia Mayo Clinic: David Warner PANDA Retrospective cohort; Neurocognitive IHR <3y v. Closed (105 sibling pairs) (US: multi-site) Prospective evaluation battery No surgery (sibs) Columbia: Lena Sun GAS Prospective, Periop outcomes; General v. Closed (722 subjects) (international: randomized Neurocognitive Awake regional RCH: Andrew Davidson multi-site) (address causality) battery BCH: Mary Ellen McCann PANDA Study • • • • Pediatric Anesthesia and NeuroDevelopment Assessment Study Multi-institutional collaborative effort. Led by Columbia University. Prospective observational trial: – Inguinal hernia repair in infants. – Sibling pairs requiring/not requiring repair as neonates. – Neurodevelopmental assessments over time. * Minimize environmental and genetic confounders. * Cannot address causality. PANDA Study Association Between a Single General Anesthesia Exposure Before Age 36 Months and Neurocognitive Outcomes in Later Childhood Lena S. Sun, MD; Guohua Li, MD, DrPH; Tonya L. K. Miller, MD; Cynthia Salorio, PhD; MaryW. Byrne, PhD, MPH; David C. Bellinger, PhD, MSc; Caleb Ing, MD, MS; Raymond Park, MD; Jerilynn Radcliffe, PhD; Stephen R. Hays, MD, MS; Charles J. DiMaggio, PhD; Timothy J. Cooper, PsyD; Virginia Rauh, ScD; Lynne G. Maxwell, MD; Ahrim Youn, PhD; Francis X. McGowan, MD JAMA. 2016;315(21):2312-2320. doi:10.1001/jama.2016.6967. “Among healthy children with a single anesthesia exposure before age 36 months, compared with healthy siblings with no anesthesia exposure, there were no statistically significant differences in IQ scores in later childhood.” GAS Study • • • • General Anaesthesia compared to Spinal anaesthesia Study Multi-national, multi-center collaborative effort. US effort led by Children’s Hospital Boston. Prospective randomized trial: – Inguinal hernia in infants. – Neuraxial (bupivacaine) versus general (sevoflurane) anesthesia. – Neurodevelopmental assessments at 2 and 5 years. Timeline 2007 2008 2009 Recruit x x x Yr 2 assessment Yr 5 assessment x 2010 2011 x x 2012 2013 2014 x x x GAS Study “FINDINGS: Outcome data were available for 238 children in the awake-regional group and 294 in the general anaesthesia group. ... There was equivalence in mean between groups (awake-regional minus general anaesthesia 0.169, 95% CI -2.30 to 2.64). INTERPRETATION: … we found no evidence that just less than 1 h of sevoflurane anaesthesia in infancy increases the risk of adverse neurodevelopmental outcome at 2 years of age compared with awake-regional anaesthesia.” Lancet. 2016 Jan 16;387(10015):239-50. doi: 10.1016/S01406736(15)00608-X. Epub 2015 Nov 4. What We Know: Animal Data • Failure to provide adequate anesthesia is causally associated with adverse neurodevelopmental outcomes in animal models and in humans. • Long-term and/or high-dose exposure to anesthetic agents induces threshold-sensitive dose-dependent apoptotic neurodegeneration and adverse behavioral sequelae in developmentally immature animals. • Short-term and/or lower-dose exposure, or exposure outside critical neurodevelopmental windows, or exposure with concomitant pain, does not. • Relevance of animal data to pediatric anesthetic practice remains unknown. What We Know: Human Data • Multiple anesthetic exposure in human infants and young children exhibits a threshold-sensitive dosedependent association with learning disability; causality is unknown. • Single brief anesthetic exposure in children is not associated with increased risk of adverse neurodevelopmental consequences, independent of patient age, former prematurity, or anesthetic technique. PANDA Symposium 2016 • Risk of multiple and/or prolonged anesthetic exposure remains unknown. What We Would Like to Do • Provide appropriate anesthesia in all settings. • Delay procedures in developmentally vulnerable children if surgically reasonable. • Consider alternatives to general anesthesia when feasible. •Minimize duration of exposure when general anesthesia cannot be avoided. • Encourage ongoing research efforts to provide more definitive data on actual risk in humans. Endoscopy3: What We Have to Do • Allow spontaneous ventilation. • Avoid airway support & instrumentation. • Share airway access in confined space. • Preserve stability despite physiologic fragility. • Support multiple providers & procedures. Anesthesia for Triple Endoscopy • Stable adequate anesthesia is challenging. • Procedural delay may not be reasonable. • Alternatives to general anesthesia not feasible. • Duration of exposure potentially prolonged. • Definitive data generally lacking: Agent? How? Challenge of Steady-State Apnea Movement Disadvantage of Bolus Dosing FINDINGS: Under inhalational anesthesia with halothane, fentanyl 0.5 mcg/kg: Apnea in 5% of patients without obstructive sleep apnea; Apnea in 50% of patient with obstructive sleep apnea (mean AHI 29.4). INTERPRETATION: “The addition of an opioid analgesic … led to central apnea in approximately half of the OSA group.” Waters KA, et al.: Effects of OSA, inhalational anesthesia, and fentanyl on the airway and ventilation of children. J Appl Physiol (1985) 2002 May;92(5):1987-94. Advantage of Continuous Infusion De Vito A, et al.: Drug-induced sleep endoscopy: conventional versus target controlled infusion techniques--a randomized controlled study. Eur Arch Otorhinolaryngol 2011 Mar;268(3):457-62. Anesthesia for Endoscopy3: VCH Mask inhalational induction (sevoflurane) v. IV induction (propofol) Propofol IV infusion for maintenance (minimize bolus dosing): takes time! Titrated supplemental agents as needed (ketamine > opioid): takes time! Topical local anesthetic (lidocaine) for airway instrumentation: takes time! Natural airway with minimal intervention for ENT (DISE, rigid) Mask > LMA > ETT for pulmonology (flexible) Airway of choice for GI (endoscopy) PATIENCE & OPEN COMMUNICATION CRITICAL! Special Thanks Dr. Thanh T. Nguyen Assistant Professor of Clinical Anesthesiology Anesthesiology - Pediatric Division Vanderbilt University Medical Center Pediatric Anesthesiologist Monroe Carell Jr. Children’s Hospital at Vanderbilt