* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Surface and colloidal chemistry
Synthetic setae wikipedia , lookup
Radiation pressure wikipedia , lookup
Nanofluidic circuitry wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Liquid crystal wikipedia , lookup
Sol–gel process wikipedia , lookup
State of matter wikipedia , lookup
Tunable metamaterial wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Nanochemistry wikipedia , lookup
Centrifugal micro-fluidic biochip wikipedia , lookup
Self-assembled monolayer wikipedia , lookup
Ultrahydrophobicity wikipedia , lookup
Surface tension wikipedia , lookup
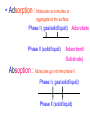
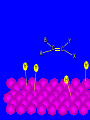
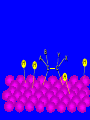
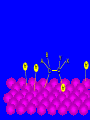
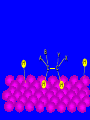
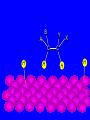
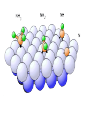
Surface and Colloidal Chemistry References: 1. Physical Chemistry, P.W. Atkins 2. Physical Chemistry Part II : Surface, Colloid and Macromolecular Chemistry, Open University of Sri Lanka Clean Surface of a Metal Surface with adsorbed species / impurity Clean Surface of a Metal Surface with adsorbed species / impurity • Surface and interface - A boundary between two phases is called a surface or interface. eg: the surface of a solid - interface is preferred for the boundary between two condensed phases e.g. the solid/gas interface - Types of Interfaces Phases in contact Examples from common use Pharmaceutical Dosage Form Gas - Gas No interface possible None Gas - liquid Surface of a drink Foams and Aerosols Gas - Solid Top of a desk Liquid - liquid Two immiscible liquids Emulsions, creams, and lotions Liquid - solid Water droplet on a glass Suspensions Solid - Solid A tire sliding on the road Powder particles inside a capsule or tablet Tablets & Capsules – Solid - Solid systems are relatively non reactive because of the nature of solids. All the other systems are very dynamic and deserve our interest. • - small open tube is inserted into a container with water(a) water raises into the tube get a liquid-gas-solid interface Mercury concave shape (meniscus) convex meniscus adhesive >> cohesive cohesive >> adhesive • The height of the liquid column (h) is related to surface tension, Radius of the tube (R), density of the liquid and angle of contact (θ) between liquid and the tube. • Forces of attraction between a liquid and a solid surface are called adhesive forces. • The difference in strength between cohesive forces and adhesive forces determine the behavior of a liquid in contact with a solid surface. • Water does not wet waxed/oily surfaces because • cohesive forces within the water drops >> the adhesive forces between the • water drops and the oily surface. • Water wets glass and spreads out on it because • • the adhesive forces between the >> cohesive forces within the liquid glass and water • What is contact angle? • Contact angle ,θ , is a quantitative measure of the wetting of a solid by a liquid. • It is defined geometrically as the angle formed by a liquid at the three phase boundary where a liquid, gas and solid intersect θ < 90 - the liquid wet the solid. θ > 90 - non-wetting/poor wetting surface. • A zero contact angle represents complete wetting. The molecules at the surface of this sample of liquid water are not surrounded by other water molecules. The molecules inside the sample are surrounded by other molecules The unbalanced attraction of molecules at the surface of a liquid tends to pull the molecules back into the bulk liquid leaving the minimum number of molecules on the surface. What is surface tension (γ) ? • Surface tension is a measurement of the cohesive energy present at an interface. • The interactions of a molecule in the bulk of a liquid are balanced by an equal attractive force in all directions. • Molecules on the surface of a liquid experience an imbalance of forces The net effect of this situation is the presence of free energy at the surface. The excess energy is called surface free energy and can be quantified • Surface tension can be defined as - Force acting on unit length of the surface which opposes the extension of the surface is called as. Surface tension = Force / length Nm-1 or - Work required to be done on a surface to extend its area by unit of area. Surface tension = work done on a surface /change in area γ = w / dA J/m2 = Nm-1 w= γ dA Therefore work done by the surface (w) = - γ dA (1) • From thermodynamics G = H – TS • dG = dH – TdS – SdT H - Enthalpy • At constant T dG = dH – TdS (2) • • • Since At Constant P H = u + pv dH = du + pdv + vdp dH = du + pdv (3) w = work done • Since dq = du + pex dv + w (4) dq = work other S – Entropy G – Gibbs energy U – Internal energy than PV work • For a reversible change • dq = TdS and Pex = P TdS = du + pdv + w • • • From (2) and (3) From (5) and (6) But • Therefore at Constant T & P pressure (5) dG = du + pdv – TdS dG = - w w = - γdA Pex = external (6) dGT,P = γdA Curved Surfaces: The liquid surface is not generally flat. Laplace equation gives the relationship between Pressure and γ for a curved surface. Consider a bubble (vapour trapped in liquid) or a droplets ( liquid in vapour) sphere - area 4πR2 Pi – pressure inside the cavity F0 Po – pressure outside the cavity Therefore outward force (F0) = 4πR2Pi Fi The force inward is arises from the external pressure Po and the surface tension. ( Fi = Po X area + FS.T). The difference in surface area is = 4π( R +dR)2 - 4πR2 dA = 8π R dR Since dw = γdA Since dw = FS.T. dR dw= 8π R dR γ FS.T. = 8πRγ At Equilibrium state Fi = F0 4πR2P0 + FS.T = 4πR2Pi 4πR2 (Pi – Po) = 8πRγ Pi – Po = 2 γ /R is The Laplace Equation Kelvin equation • The relationship between the vapour pressure outside a spherical droplet to its radius and the surface tension is given by Kelvin equation. • Pr Pr 2 Mγ ln P∞ P∞ = RTρr Pr – the vapour pressure outside a spherical droplet of radius r P∞ - vapour pressure outside a plane surface γ – surface tension, R- gas constant, ρ- density of the liquid M- molecular weight Pr 〉0 P∞ And T Pr > P∞ Pr and r Pr Factors affecting surface tension • Surface tension • - is the energy that is required to stretch the surface of a liquid by unity • - requires an input of energy: • 1. Temperature • The stretching of a surface of a warmer liquid is easier because the molecules at the surface are "hopping around" more. • - γ of most liquids always decreases with increasing • temperature. At high T attractive forces of the liquid molecules 2. Added solute : • Surface tension of a liquid is highly depend on the solutes/ impurities dissolved in the liquid. • Therefore γ depends upon what substances (molecules) are at or near the surface of that liquid. • Since the "surface" is a boundary of a very thin thickness, surface tension is a sensitive function of impurities, solutes, pH, and anything else that can fit at or near the surface. • The dissolved solutes could increase or decrease the surface tension of a pure liquid: • γ • γ surface/ capillary active agents surface / capillary inactive agents • Capillary active agents: • Eg: Organic acids, alcohols, esters, ethers, amines, ketones • - contains polar and non polar groups • - prefer to concentrate on the water surface • - hydrophilic group is directed into the water and the • hydrophobic group is directed away from the water • - molecular concentration is higher on the surface than in • the bulk • Solute-solvent interactions < solvent- solvent interactions • • Capillary inactive agents: • - increase the surface tension of the water/liquid • Eg. Ionic salts, sugar, glycerin • - Concentration in the bulk > concentration at the interface • - Solute-solvent interactions > solvent- solvent interactions • Adhesive forces > cohesive forces • There is another group of solutes which adsorbed to the water surface even more strongly than surface active agents : referred as surfactants. • - decreased γ rapidly • Surfactants are among the most widely used groups of chemicals in the world. • Eg: • - detergents • - soaps • - emulsifiers • - wetting agents • • Soaps: are akaline carboxylate salts of long chain fatty acids RCO2-Na+ • • Detergents: are sodium sulphonate salts of long chain sulphonic acids • RSO3-Na+ • Adsorption : Molecules accumulate or • aggregate at the surface Phase I ( gas/solid/liquid) • • Phase II (solid/liquid) Adsrobate Adsorbent/ Substrate) • Absoption : Molecules go into the phase II Phase I ( gas/solid/liquid) Phase II (solid/liquid) • Adsorption - the process in which a molecule becomes adsorbed onto a surface of another phase • Adsorbate - atomic or molecular species which are adsorbed (or are capable of being adsorbed) onto the substrate • Substrate/adsorbent - the solid surface onto which adsorption can occur Molecular Adsorption • The adsorption of molecules on to a surface is a necessary prerequisite to any surface involved chemical process. • For example, in the case of a surface catalyzed reaction the following steps should occur • • • • • • - Diffusion of reactants to the active surface - Adsorption of one or more reactants onto the surface - Surface reaction - Desorption of products from the surface - Diffusion of products away from the surface Mechanism of Catalytic Hydrogenation B H H A H H Y C C X B A H Y C C X H H H B H Y A H C X C H H B H Y A H C X C H H B H Y A C H X C H H B Y A C H H X C H H How do Molecules Bond to Surfaces ? • There are two principal modes of adsorption of molecules on surfaces : • Physical Adsorption (Physisorption ) • Chemical Adsorption (Chemisorption) • Chemisorption is adsorption in which the forces involved are valence forces • Physisorption is adsorption in which the forces involved are intermolecular forces (van der Waals forces) Chemisorption Physisorption - unlimited (but a given molecule may effectively adsorb only over a small range) Near or below the condensation point of the gas (e.g. Xe < 100 K, CO2 < 200 K) Wide range (related to the chemical bond strength) - typically 40 - 800 kJ mol-1 Related to factors like molecular mass and polarity but typically 5-40 kJ mol-1 Crystallographic Specificity (variation between different surface planes of the same crystal) Marked variation between crystal planes Virtually independent of surface atomic geometry Nature of Adsorption Often dissociative May be irreversible Non-dissociative Reversible Saturation Uptake Limited to one monolayer Multilayer uptake possible Kinetics of Adsorption Very variable - often an activated process Fast - since it is a non-activated process Temperature Range (over which adsorption occurs) Adsorption Enthalpy Geometry of Ni(100)+Ag(111) multilayers • Adsorption of N2 on Fe surface • At 78 K Liquid nitrogen is adsorbed physically on Fe as N2 molecules • • T [N2 adsorbed] • At room temp. N2 does not adsorbed on Fe at all. • However at 750 K N2 is chemisorbed on Fe surfaces as N atoms