* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download NIZORAL 200 MG TABLETS
Environmental impact of pharmaceuticals and personal care products wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Orphan drug wikipedia , lookup
Drug discovery wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Prescription drug prices in the United States wikipedia , lookup
Tablet (pharmacy) wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Neuropharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Prescription costs wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Dydrogesterone wikipedia , lookup
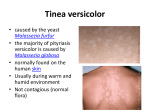
1 8.12 אושר ""פורמט עלון זה נקבע ע"י משרד הבריאות ותוכנו נבדק ואושר 1. TRADE NAME OF THE MEDICINAL PRODUCT NIZORAL 200 MG TABLETS 2. QUALITATIVE AND QUANTITATIVE COMPOSITION Each tablet contains 200 mg ketoconazole. For excipients, see section 6.1. 3. PHARMACEUTICAL FORM White, circular, flat bevel-edged, half-scored tablet with the inscription “JANSSEN” on one side and “K/200” on the reverse. 4. CLINICAL PARTICULARS 4.1. Therapeutic indications Because of the risk for serious hepatic toxicity, NIZORAL tablets should be used only when the potential benefits are considered to outweigh the potential risks, taking into consideration the availability of other effective antifungal therapy. Indications are: Infections of the skin, hair, and mucosa, induced by dermatophytes and/or yeasts that cannot be treated topically because of the site or the extent of the lesion or deep infection of the skin. - Dermatophytosis - Pityriasis versicolor - Pityrosporum folliculitis L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 2 - Cutaneous candidosis - Chronic mucocutaneous candidosis - Oropharyngeal and esophageal candidosis - Chronic, recurrent vaginal candidosis Systemic fungal infections Ketoconazole does not penetrate well in the CNS. Therefore, fungal meningitis should not be treated with oral ketoconazole. 4.2. - Paracoccidioidomycosis - Histoplasmosis - Coccidioidomycosis - Blastomycosis Posology and method of administration NIZORAL should be taken during meals for maximal absorption. - Infections of the skin, hair, and mucosa induced by dermatophytes and/or yeasts, and systemic infections, that cannot be treated topically because of the site or the extent of the lesion or deep infection of the skin: Adults One tablet (= 200 mg) once daily with a meal. When no adequate response is obtained with this dose, the dose should be increased to 2 tablets (= 400 mg). once daily. -Adults with vaginal candidosis: two tablets (= 400 mg) once daily with a meal. Children - Children weighing from 15 to 30 kg: half a tablet (=100 mg) once daily with a meal. - Children weighing more than 30 kg: same as for adults. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 3 The usual duration of treatment is: - Vaginal candidosis: 5 consecutive days; - Skin mycosis induced by dermatophytes: approximately 4 weeks; - Pityriasis versicolor: 10 days; - Oral and skin mycosis induced by Candida: 2-3 weeks; - Hair infections: 1-2 months; - Paracoccidioidomycosis, histoplasmosis, coccidioidomycosis: the usual duration of therapy is 6 months. For all indications, treatment should be continued without interruption until clinical parameters or laboratory tests indicate that the fungal infection has resolved. An inadequate treatment period may lead to recurrence of the active infection. However, treatment should be stopped immediately and liver function testing should be conducted when signs and symptoms suggestive of hepatitis such as anorexia, nausea, vomiting, fatigue, jaundice, abdominal pain or dark urine occur. Special Patient Population: Hepatic Impairment (see section 4.3 Contraindications) 4.3. Contraindications NIZORAL tablets are contraindicated in the following situations: - In patients with a known hypersensitivity to ketoconazole or to any of the excipients. - In patients with acute or chronic liver disease. - Coadministration of a number of CYP3A4 substrates is contraindicated with NIZORAL Tablets. Increased plasma concentrations of these drugs, caused by coadministration with ketoconazole, may increase or prolong both therapeutic and adverse effects to such an extent that a potentially serious situation may occur. For example, increased plasma concentrations of some of these drugs can lead to QT prolongation and ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. Specific examples are listed in Section 4.5 Interaction with other medicinal products and other forms of interaction. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 4 4.4. Special warnings and special precautions for use Because of the risk for serious hepatotoxicity, NIZORAL tablets should be used only when the potential benefits are considered to outweigh the potential risks, taking into consideration the availability of other effective antifungal therapy. Assess liver function, prior to treatment to rule out acute or chronic liver disease, and monitor at frequent and regular intervals during treatment, and at the first signs or symptoms of possible hepatotoxicity. Hepatotoxicity Very rare cases of serious hepatotoxicity including cases with a fatal outcome or requiring liver transplantation have occurred with the use of oral ketoconazole (see section 4.8 Undesirable effects). Some patients had no obvious risk factors for liver disease. Cases have been reported that occurred within the first month of treatment, including some within the first week. The cumulative dose of the treatment is a risk factor for serious hepatotoxicity. Monitor liver function in all patients receiving treatment with NIZORAL tablets (see Monitoring of hepatic function). Patients should be instructed to promptly report to their physician signs and symptoms suggestive of hepatitis such as anorexia, nausea, vomiting, fatigue, jaundice, abdominal pain or dark urine. In these patients, treatment should be stopped immediately and liver function testing should be conducted. Monitoring of hepatic function Monitor liver function in all patients receiving treatment with NIZORAL tablets. Monitor liver function prior to treatment to rule out acute or chronic liver disease (see 4.3 Contraindications), at frequent and regular intervals during treatment, and at the first signs or symptoms of possible hepatic toxicity. When the liver function tests indicate liver injury, the treatment should be stopped immediately. In patients with elevated liver enzymes, or who have experienced liver toxicity with other drugs, treatment should not be started unless the expected benefit exceeds the risk of hepatic injury. In such cases close monitoring of the liver enzymes is necessary. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 5 Monitoring of adrenal function In volunteers on daily doses of 400 mg and more, ketoconazole has been shown to reduce the cortisol response to ACTH stimulation. Therefore, adrenal function should be monitored in patients with adrenal insufficiency or with borderline adrenal function, in patients under prolonged periods of stress (major surgery, intensive care, etc.), and in patients on prolonged therapy presenting signs and symptoms suggestive of adrenal insufficiency. Pediatric use Documented use of NIZORAL tablets in children weighing less than 15 kg is very limited. Therefore, it is not recommended to administer NIZORAL tablets to small children. Reduced gastric acidity When the gastric acidity is reduced, absorption of ketoconazole from ketoconazole tablets is reduced. In subjects with reduced gastric acidity, whether from disease (e.g. subjects with achlorhydria) or from concomitant medication (e.g. subjects taking drugs that reduce gastric acidity), it is advisable to administer NIZORAL Tablets with an acidic beverage (such as non-diet cola). The antifungal activity should be monitored and the ketoconazole dose increased as deemed necessary. See Section 4.5 Interaction with other medicinal products and other forms of interaction, Drugs that may decrease ketoconazole plasma concentrations and Section 5.2 Pharmacokinetic properties/Absorption. Drug interaction potential Coadministration of specific drugs with ketoconazole may result in changes in efficacy of ketoconazole and/or the coadministered drug, life-threatening effects and/or sudden death. Drugs that are contraindicated, not recommended or should be used with caution in combination with ketoconazole are listed in see section 4.5. Interaction with other medicinal products and other forms of interaction. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 6 4.5. Interaction with other medicinal products and other forms of interaction Ketoconazole is mainly metabolized through CYP3A4. Other substances that either share this metabolic pathway or modify CYP3A4 activity may influence the pharmacokinetics of ketoconazole. Similarly, ketoconazole may modify the pharmacokinetics of other substances that share this metabolic pathway. Ketoconazole is a potent CYP3A4 inhibitor and a Pglycoprotein inhibitor. When using concomitant medication, the corresponding label should be consulted for information on the route of metabolism and the possible need to adjust dosages. Interaction studies have only been performed in adults. The relevance of the results from these studies in pediatric patients is unknown. • Drugs that may decrease ketoconazole plasma concentrations Drugs that reduce the gastric acidity (e.g. acid neutralizing medicines such as aluminium hydroxide, or acid secretion suppressors such as H2-receptor antagonists and proton pump inhibitors) impair the absorption of ketoconazole from ketoconazole tablets. These drugs should be used with caution when coadministered with ketoconazole tablets: Ketoconazole should be administered with an acidic beverage (such as nondiet cola) upon co-treatment with drugs reducing gastric acidity. Acid neutralizing medicines (e.g. aluminium hydroxide) should be administered at least 1 hour before or 2 hours after the intake of NIZORAL Tablets. Upon coadministration, the antifungal activity should be monitored and the ketoconazole dose increased as deemed necessary. Coadministration of ketoconazole with potent enzyme inducers of CYP3A4 may decrease the bioavailability of ketoconazole to such an extent that efficacy may be largely reduced. Examples include: L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 7 • Antibacterials: isoniazid, rifabutin, rifampicin. • Anticonvulsants: carbamazepine (see also under ‘Drugs that may have their plasma concentrations increased’), phenytoin. • Antivirals: efavirenz, nevirapine. Therefore, administration of potent enzyme inducers of CYP3A4 with ketoconazole is not recommended. The use of these drugs should be avoided from 2 weeks before and during treatment with ketoconazole, unless the benefits outweigh the risk of potentially reduced ketoconazole efficacy. Upon coadministration, the antifungal activity should be monitored and the ketoconazole dose increased as deemed necessary. • Drugs that may increase ketoconazole plasma concentrations Potent inhibitors of CYP3A4 (e.g. antivirals such as ritonavir, ritonavirboosted darunavir and ritonavir-boosted fosamprenavir) may increase the bioavailability of ketoconazole. These drugs should be used with caution when coadministered with ketoconazole tablets. Patients who must take ketoconazole concomitantly with potent inhibitors of CYP3A4 should be monitored closely for signs or symptoms of increased or prolonged pharmacologic effects of ketoconazole, and the ketoconazole dose should be decreased as deemed necessary. When appropriate, ketoconazole plasma concentrations should be measured. • Drugs that may have their plasma concentrations increased by ketoconazole Ketoconazole can inhibit the metabolism of drugs metabolized by CYP3A4 and can inhibit the drug transport by P-glycoprotein, which may result in increased plasma concentrations of these drugs and/or their active metabolite(s) when they are administered with ketoconazole. These elevated plasma concentrations may increase or prolong both therapeutic and adverse effects of these drugs. CYP3A4-metabolized drugs known to prolong the QT interval may be contraindicated with ketoconazole, since the combination may lead to ventricular tachyarrhythmias including occurrences of torsade de pointes, a potentially fatal arrhythmia. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 8 The interacting drugs are categorized as follows: − ‘Contraindicated’: Under no circumstances should the drug be coadministered with ketoconazole, and up to one week after discontinuation of treatment with ketoconazole. − ‘Not recommended’: The use of the drug should be avoided during and up to one week after discontinuation of treatment with ketoconazole, unless the benefits outweigh the potentially increased risks of side effects. If coadministration cannot be avoided, clinical monitoring for signs or symptoms of increased or prolonged effects or side effects of the interacting drug is recommended, and its dosage should be reduced or interrupted as deemed necessary. When appropriate, plasma concentrations should be measured. − ‘Use with caution’: Careful monitoring is recommended when the drug is coadministered with ketoconazole. Upon coadministration, patients should be monitored closely for signs or symptoms of increased or prolonged effects or side effects of the interacting drug, and its dosage should be reduced as deemed necessary. When appropriate, plasma concentrations should be measured. Examples of drugs that may have their plasma concentrations increased by ketoconazole presented by drug class with advice regarding coadministration with ketoconazole: Drug Class Contraindicated Alpha Blockers Not Recommended Use with Caution tamsulosin Analgesics levacetylmethadol (levomethadyl), methadone Antiarrhythmics disopyramide, dofetilide, fentanyl alfentanil, buprenorphine IV and sublingual, oxycodone digoxin L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 9 Drug Class Contraindicated Not Recommended Use with Caution dronedarone, quinidine Antibacterials rifabutin Anticoagulants and Antiplatelet Drugs rivaroxaban Anticonvulsants carbamazepine Antidiabetics coumarins, cilostazol repaglinide, saxagliptin Antihelmintics and Antiprotozoals halofantrine praziquantel Antihistamines astemizole, mizolastine, terfenadine ebastine Antimigraine Drugs ergot alkaloids, such as dihydroergotamine, ergometrine (ergonoine), ergotamine, methylergometrine (methylergonovine) eletriptan Antineoplastics irinotecan dasatinib, nilotinib, trabectedin bortezomib, busulphan, docetaxel, erlotinib, imatinib, ixabepilone, lapatinib, trimetrexate, L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 10 Drug Class Contraindicated Not Recommended Use with Caution vinca alkaloids Antipsychotics, Anxiolytics and Hypnotics lurasidone, oral midazolam, pimozide, sertindole, triazolam alprazolam, aripiprazole, brotizolam, buspirone, haloperidol, midazolam IV, perospirone, quetiapine, ramelteon, risperidone Antivirals maraviroc, indinavir, saquinavir Beta Blockers nadolol Calcium Channel Blockers bepridil, felodipine, lercanidipine, nisoldipine other dihydropyridine s, including verapamil Cardiovascular Drugs, Miscellaneous ivabradine, ranolazine aliskiren Diuretics eplerenone Gastrointestinal Drugs cisapride, domperidone Immunosuppressants aprepitant everolimus budesonide, ciclesonide, cyclosporine, dexamethasone, L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 11 Drug Class Contraindicated Not Recommended Use with Caution fluticasone, methylpredniso lone, rapamycin (also known as sirolimus), tacrolimus, temsirolimus Lipid Regulating Drugs lovastatin, simvastatin Respiratory Drugs atorvastatin salmeterol SSRIs, Tricyclics and Related Antidepressants reboxetine Urological Drugs Other colchicine, in subjects with renal or hepatic impairment vardenafil fesoterodine. imidafenacin, sildenafil, solifenacin, tadalafil, tolterodine colchicine *alcohol, alitretinoin (oral formulation), cinacalcet, mozavaptan, tolvaptan L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 12 *Exceptional cases have been reported of a disulfiram-like reaction to alcohol, characterized by flushing, rash, peripheral oedema, nausea and headache. All symptoms completely resolved within a few hours. 1.1. Pregnancy and lactation 4.6.1. Use during pregnancy There is limited information on the use of NIZORAL tablets during pregnancy. Animal studies have shown reproductive toxicity (see section 5.3 Preclinical safety data). The potential risk to humans is unknown. Therefore, NIZORAL tablets should not be used during pregnancy unless the potential benefit to the mother outweighs the possible risk to the foetus. 4.6.2. Use during lactation Since ketoconazole is excreted in the milk, mothers who are under treatment should not breast-feed. 1.2. Effects on ability to drive and use machines No effects have been observed. 1.3. Undesirable effects 4.8.1. Clinical trial data The safety of NIZORAL Tablets was evaluated in 4735 subjects in 92 clinical trials where NIZORAL Tablets were administered to treat a fungal infection or to healthy volunteers. Adverse drug reactions that were reported ≥1% of NIZORAL Tablets-treated subjects are shown in Table 1. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 13 Table 1. Adverse Drug Reactions Reported in ≥1% of 4735 NIZORAL Tablets-treated Subjects in 92 Clinical Trials System Organ Class % Preferred Term Gastrointestinal Disorders Abdominal pain 1.2 Diarrhoea 1.8 Nausea 2.5 Hepato-biliary Disorders Hepatic function abnormal 1.2 Nervous System Disorders Headache 2.4 Additional adverse drug reactions that occurred in <1% of NIZORAL Tablets-treated subjects in the clinical datasets are listed in Table 2. Table 2. Adverse Drug Reactions Reported in < 1% of 4735 NIZORAL Tablets-treated Subjects in 92 Clinical Trials System Organ Class Preferred Term Endocrine Disorders Gynaecomastia Eye Disorders Photophobia Gastrointestinal Disorders Abdominal pain upper Constipation Dry mouth Dysgeusia Dyspepsia Flatulence Tongue discolouration Vomiting General Disorders and Administration Site Conditions Asthenia Chills Fatigue L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 14 Hot flush Malaise Oedema peripheral Pyrexia Hepato-biliary Disorders Hepatitis Jaundice Immune System Disorders Anaphylactoid reaction Investigations Platelet count decreased Metabolism and Nutrition Disorders Alcohol intolerance Anorexia Hyperlipidaemia Increased appetite Musculoskeletal and Connective Tissue Disorders Myalgia Nervous System Disorders Dizziness Paraesthesia Somnolence Psychiatric Disorders Insomnia Nervousness Reproductive System and Breast Disorders Menstrual disorder Respiratory, Thoracic and Mediastinal Disorders Epistaxis Skin and Subcutaneous Tissue Disorders Alopecia Dermatitis Erythema Erythema multiforme Pruritus Rash Urticaria Xeroderma L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 15 Vascular Disorders Orthostatic hypotension 4.8.2. Post-marketing experience Very common ≥1/10 Common ≥1/100 and < 1/10 Uncommon ≥1/1,000 and <1/100 Rare ≥1/10,000 and <1/1,000 Very rare <1/10,000, including isolated reports In Table 3, ADRs are presented by frequency category based on spontaneous reporting rates. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 16 Table 3. Adverse Drug Reactions Identified During Postmarketing Experience with NIZORAL Tablets by Frequency Category Estimated from Spontaneous Reporting Rates Blood and Lymphatic System Disorders Very rare thrombocytopenia; Immune System Disorders Very rare allergic conditions including anaphylactic shock, anaphylactic reaction and angioneurotic oedema Endocrine Disorders Very rare adrenocortical insufficiency Nervous System Disorders Very rare reversible intracranial pressure increased (e.g. papilloedema, fontanelle bulging in infants) Hepato-biliary Disorders Very rare serious hepatotoxicity, including hepatitis cholestatic, biopsyconfirmed hepatic necrosis, cirrhosis, hepatic failure including cases resulting in transplantation or death. (see section 4.4 Special warnings and special precautions for use) Skin and Subcutaneous Tissue Disorders Very rare acute generalised exanthematous pustulosis, photosensitivity Musculoskeletal and Connective Tissue Disorders Very rare arthralgia Reproductive System and Breast Disorders Very rare erectile dysfunction ; with doses higher than the recommended therapeutic dose of 200 or 400mg daily azoospermia, 4.9 Overdose There is no known antidote to ketoconazole. Symptoms: Adverse drug reactions reported by patients taking high doses of NIZORAL are available in 6 clinical trials in a total of 459 patients where NIZORAL was administered at doses of 1,200 mg daily either in tablet form or as an oral suspension. The most commonly reported adverse drug reactions were nausea (27.2%), fatigue (including somnolence and lethargy) (14.2%), vomiting (12.6%), gastrointestinal pain (including abdominal discomfort, gastrointestinal disorder, stomach discomfort) (12.0%), anorexia (including weight decreased, decreased appetite) (7.4%), flushing (including L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 17 hyperhidrosis) (6.3%), oedema (5.7%), gynaecomastia (4.8%), rash (including eczema, purpura, dermatitis) (3.3%), diarrhoea (2.2%), headache (2.0%), dysgeusia (1.3%), and alopecia (1.1%). Treatment: In the event of acute accidental overdose, treatment consists of supportive and symptomatic measures. Within the first hour after ingestion, activated charcoal may be administered. 5 PHARMACOLOGICAL PROPERTIES 5.1 Pharmacodynamic properties Pharmacotherapeutic classification: Antimycotics for systemic use, imidazole derivatives ATC code: J02A B02 Ketoconazole is a synthetic imidazole dioxolane derivative with a fungicidal or fungistatic activity against dermatophytes, yeasts (Candida, Malassezia, Torulopsis, Cryptococcus ), dimorphic fungi and eumycetes. Less sensitive are: Aspergillus spp., Sporothrix schenckii, some Dematiaceae, Mucor spp. and other phycomycetes, except Entomophthorales. Ketoconazole inhibits the biosynthesis of ergosterol in fungi and changes the composition of other lipid components in the membrane. Data from some clinical PK/PD studies and drug interaction studies suggest that oral dosing with ketoconazole at 200 mg twice daily for 3-7 days can result in a small increase of the QTc interval: a mean maximum increase of about 6 to 12 msec was seen at ketoconazole peak plasma levels, about 1-4 hours after ketoconazole administration. This small prolongation of the QTc interval, however, is not considered to be clinically relevant. At the therapeutic dosage of 200 mg once daily, a transient decrease in the plasma concentrations of testosterone can be observed. Testosterone concentrations return to pre-dose concentrations within 24 hours after administration of ketoconazole. During long-term therapy at this dosage, testosterone concentrations are usually not significantly different from controls. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 18 In volunteers on daily doses of 400 mg and more, ketoconazole has been shown to reduce the cortisol response to ACTH stimulation (see section 4.4 Special warnings and special precautions for use). 5.2 Pharmacokinetic properties Absorption Ketoconazole is a weak dibasic agent and thus requires acidity for dissolution and absorption. Mean peak plasma concentrations of approximately 3.5 µg/mL are reached within 1 to 2 hours, following oral administration of a single 200 mg dose taken with a meal. Oral bioavailability is maximal when the tablets are taken with a meal. Absorption of ketoconazole tablets is reduced in subjects with reduced gastric acidity, such as subjects taking medications known as acid neutralizing medicines (e.g. aluminium hydroxide) and gastric acid secretion suppressors (e.g. H2-receptor antagonists, proton pump inhibitors) or subjects with achlorhydria caused by certain diseases. (See Section 4.4 Special warnings and special precautions for use and Section 4.5 Interaction with other medicinal products and other forms of interaction.) Absorption of ketoconazole under fasted conditions in these subjects is increased when ketoconazole tablets are administered with an acidic beverage (such as nondiet cola). After pretreatment with omeprazole, a proton pump inhibitor, the bioavailability of a single 200-mg dose of ketoconazole under fasted conditions was decreased to 17% of the bioavailability of ketoconazole administered alone. When ketoconazole was administered with non-diet cola after pretreatment with omeprazole, the bioavailability was 65% of that after administration of ketoconazole alone. Distribution In vitro, the plasma protein binding is about 99% mainly to the albumin fraction. Ketoconazole is widely distributed into tissues; however, only a negligible proportion reaches the cerebrospinal fluid. Metabolism Following absorption from the gastrointestinal tract, ketoconazole is converted into several inactive metabolites. In vitro studies have shown that CYP3A4 is the major enzyme involved in the metabolism of ketoconazole. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 19 The major identified metabolic pathways are oxidation and degradation of the imidazole and piperazine rings, by hepatic microsomal enzymes. In addition, oxidative O-dealkylation and aromatic hydroxylation does occur. Ketoconazole has not been demonstrated to induce its own metabolism. Elimination Elimination from plasma is biphasic with a half-life of 2 hours during the first 10 hours and 8 hours thereafter. Approximately 13% of the dose is excreted in the urine, of which 2 to 4% is unchanged drug. The major route of excretion is through the bile into the intestinal tract with about 57% being excreted in the feces. Special Populations Patients with Hepatic or Renal Impairment In patients with hepatic or renal insufficiency the overall pharmacokinetics of ketoconazole was not significantly different when compared with healthy subjects. Pediatric Patients Limited pharmacokinetic data are available on the use of ketoconazole tablets in the pediatric population. Measurable ketoconazole plasma concentrations have been observed in preterm infants (single or daily doses of 3 to 10 mg/kg) and in pediatric patients 5 months of age and older (daily doses of 3 to 13 mg/kg) when the drug was administered as a suspension, tablet or crushed tablet. Limited data suggest that absorption may be greater when the drug is administered as a suspension compared to a crushed tablet. Conditions that raise gastric pH may lower or prevent absorption (see Section 4.4 Special warnings and special precautions for use and Section 4.5 Interaction with other medicinal products and other forms of interaction). Maximum plasma concentrations occurred 1 to 2 hours after dosing and were in the same general range as those seen in adults who received a 200-400 mg dose. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 20 5.3 Preclinical safety data Ketoconazole has been tested in a standard battery of non-clinical safety studies. Hepatotoxic effects were seen in a 12-month repeated dose dog study. Slight pathological changes in the kidney, adrenals and ovaries were noted in an 18-month repeated dose rat study. In addition, female rats showed an increase in bone fragility. The No Observed Adverse Effect Level (NOAEL) in both these studies was 10 mg/kg/day. In reproduction studies, at very high, maternally toxic doses (80 mg/kg/day and higher), ketoconazole impaired female fertility in the rat, and produced embryotoxic and teratogenic (oligodactylia and syndactylia) effects in pups. At 40 mg/kg in rats and rabbits, ketoconazole was devoid of embryotoxicity, teratogenicity and effects on fertility. No teratogenic effects were observed in mice at any dose level tested up to a maximum of 160 mg/kg. Ketoconazole is not carcinogenic or genotoxic. Electrophysiological studies have shown that ketoconazole inhibits the rapidly activating component of the cardiac delayed rectifier potassium current, prolongs the action potential duration, and may prolong the QT interval. 6. PHARMACEUTICAL PARTICULARS 6.1 List of excipients The inactive ingredients of the tablets are maize starch, lactose monohydrate, povidone, microcrystalline cellulose, silicon dioxide and magnesium stearate 6.4 Special precautions for storage Do not Store above 30° C. The tablets must be stored in a dry place. Keep out of reach of children. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc 21 Manufacturer Janssen Cilag, Latina, Italy Registration Holder J-C Health care Ltd. Kibbutz Shefayim, 60990. L:\RND\Mdreg\Leaflets\Nizoral Tabs\Aug 2012\Approved\Nizoral_Tabs_PI_Aug12_CL.doc