* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
X-ray fluorescence wikipedia , lookup
Ferromagnetism wikipedia , lookup
Wave–particle duality wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
Vibrational analysis with scanning probe microscopy wikipedia , lookup
Ultraviolet–visible spectroscopy wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Astronomical spectroscopy wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Rigid rotor wikipedia , lookup
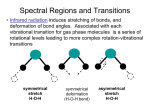
Molecules Molecular Spectra Molecular Spectra is observed when the emitting substance is in the molecular state. With high resolving power instrument , the molecular spectra disclose a three fold structure. The three type of bands are: Electronic, Vibrational, or Rotational spectrum Erot < Evib < Eelec hirarchy. For a diatomic molecule, the electronic states can be represented by plots of potential energy as a function of internuclear distance. Electronic transitions are vertical or almost vertical lines on such a plot since the electronic transition occurs so rapidly that the internuclear distance can't change much in the process. Vibrational transitions occur between different vibrational levels of the same electronic state. Rotational transitions occur mostly between rotational levels of the same vibrational state, although there are many examples of combination vibration-rotation transitions for light molecules. Rotational Energy Levels Incident electromagnetic waves can excite the rotational levels of molecules provided they have an electric dipole moment. The electromagnetic field exerts a torque on the molecule. The spectra for rotational transitions of molecules is typically in microwave region of the electromagnetic spectrum. The rotational energies for rigid molecules can be found with the the aid of the Shrodinger equation. For a diatomic molecule the rotational energy is where J is the rotational angular momentum quantum number and I is the moment of inertia The rotational constant B enables to calculate the bond length R. Rotational Transitions The allowed transitions for the diatomic molecule are regularly spaced at interval 2B. The measurement and identification of one spectral line allows one to calculate the moment of inertia and then the bond length. For a rigid rotor diatomic molecule, the selection rules for rotational transitions are ΔJ = +/-1 Vibrational Spectra of Diatomic Molecules The lowest vibrational transitions of diatomic molecules approximate the quantum harmonic oscillator and can be used to imply the bond force constants for small oscillations. A hamiltonian with a parabolic potential energy is characteristic to a harmonic oscillator with 1 Ev v 2 k 1 2 with = 0, 1, 2,…. Is vibrational quantum number The selection rules are Δ = +/-1 Vibration-Rotation Spectra Infrared spectroscopy concerns changes of vibrational and rotational state, without change of electronic state. Hence, the infrared spectrum is a vibration-rotation spectrum. The selection rules are: Δn = ± 1 ΔJ = ± 1 Considering only transitions that involve the ground vibration state, the energy change is: Raman Effect First postulated by Smekal in 1923 Dr. Chandrasekhara Venkata Raman First observation of Raman Scattering Discovered by the Indian scientist Sir C. V. Raman (1928, together with K. S. Krishnan). Raman won the Nobel Prize in Physics in 1930 for this discovery Electric dipole moment For a single dipole with a distance, d, between charges – q and +q, the dipole moment is: p = qd Heteronuclear diatomics and asymmetric triatomics: CO, NO, H2O- Have permanent dipole moment: Homonuclear diatomics: O2 , N2 No permanent dipole moment Induced dipole moment A dipole moment may be created through: • electronic excitation • the polarizability of the molecule in the presence of an electric field Induced dipole moment: p=αE α: molecular polarizability Ε: electric field (polarization = vector sum of the dipole moments per unit volume) Raman spectrum scattering Raman Stokes line – scattered photon give up energy Raman Anti-stokes line – scattered photon gain energy Light intensity Rayleigh line – elastic Schematic diagram of the process Schematic diagram of the Raman scattering process The vertical direction represents energy