* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Plateau principle wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Drug design wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
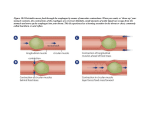
FUN2: 11:00-12:00 Scribe: Teresa Kilborn Monday, December 1, 2008 Proof: Joan-Marie Manolakis Dr. Prasain Pharmacology Page 1 of 4 Pharmacokinetics-Absorption & Distribution GIT- Gastrointestinal Tract I. Title [S1] II. What is Pharmacokinetics (PK) [S2] a. The standard dose of a drug is different from person to person depending on the age and pathophysiological condition of that person b. Ex. The standard dose of a drug is to (inaudible). The dose of a person with a heart attack will be different from other people. c. The response of our body to drugs is different. d. What is pharmacokinetics? i. How the body responds to the drug ii. How the drug responds to our body- the dynamics of the drug within the biological system e. Once a drug is introduced into our body, it undergoes several events: i. Absorption ii. Distribution- to different compartments iii. Metabolism iv. Elimination f. One of the important things is that a drug is a chemical substance which elicits a biological action, at the same time another characteristic of a drug is that it needs to be eliminated III. ADME of drugs [S3] a. Important- All chemicals are not drugs and drugs are chemicals b. Administration: i. One characteristic of a drug is that it is a chemical substance that needs to be administered at the site away from its target (i.e. organ) where it actually acts in a biological system. ii. There are several routes or ways of drug administration. iii. There are several factors, including drug type, that determine the best way to administer the drug. c. Absorption: i. The transportation of a drug from the site of entry to the site of action is absorption. ii. It varies from person to person drug to drug. d. Distribution and metabolism: i. The drug, once absorbed, is distributed into different compartments of our body and also metabolized. ii. Drugs are active molecules that have some biological effect, but drugs are metabolically inactivated and metabolism of the drug activates them. iii. Starting with the intestinal epithelium and the kidney IV. How are Drugs Admininstered? [S4]- SKIPPED V. Enteral route and Parenteral route [S5] a. Enteral route – the GIT route (oral, sublingual, or rectal) i. Advantages: 1. Convenient- anyone can take a drug, we don’t need a nurse or a doctor 2. Economic 3. Safe- most people don’t know how to inject a drug ii. Limitations: 1. First pass- metabolism effect-consequently the bioavailability is less than the parenteral route 2. Some drugs are unstable in the GIT. For example, drugs that cannot withstand the gastric acidic environment b. Parenteral- outside the GIT tract (i.e. intravenous, subcutaneous, intramuscular) i. Drugs that are poorly absorbed by the GIT or that are unstable in the GIT ii. Ex. Insulin, penicillin G (a beta lactam that is unstable in an acidic environment, thus penicillin G is not preferred in enteral administration. It is given by IV.) iii. In unconscious patients, it is hard to give drugs orally and IV or SC (parenteral administration) is preferred. iv. In emergency situations, it is also preferred because of the immediate onset of action v. Limitations: 1. Not convenient compared to oral (you must have someone with expertise) 2. You must have sterile conditions/setting 3. If you have to inject frequently, it can be hard to find a vein (this is part of not convenient) c. Both enteral and parenteral have advantages and disadvantages VI. Factors affecting oral absorption of a drug [S6] a. There are several drugs taken orally –Ex. is Aspirin. b. Once you take it orally, the absorption can be different. FUN2: 11:00-12:00 Scribe: Teresa Kilborn Monday, December 1, 2008 Proof: Joan-Marie Manolakis Dr. Prasain Pharmacology Page 2 of 4 c. The physical chemical form of the drug is important because it determines the extent of absorption. d. Before absorption, the drug is disintegrated. It dissolves in the fluid of the GIT. This is important. That is why if you take the same amount of a drug in solid form, suspension form, or solution form, the kinetics could be different. e. The extent of absorption or bioavailability is governed by several factors that are important to consider with oral absorption: i. The chemical stability of a drug ii. Dissolution iii. Presence of and type of food iv. Gastric emptying time 1. Which part of the GIT is the site of major absorption? Intestine compared to stomach. 2. The time from the stomach to intestine is the exit time. 3. Because the intestine is a major site of absorption, that is a limiting step. 4. The factor is how quickly you release the drug from stomach to intestine. 5. If a physician prescribes a certain drug it can have a very fast gastric emptying time. v. pH vi. Transport 1. Can be active or passive. 2. Basic transport of drugs: a. Most drug-like molecules are hydrophobic (in nature everything has exceptions) b. There are few criteria for drug-like molecules: Molecular weight (less than 1,000), there are a few water soluble and lipid soluble, and there are a few exceptions, but most drugs fall into lipophilic c. They can undergo basic diffusion i. Through the barrier of the intestine where a drug is absorbed. ii. The lipophilic, less polar, non ionic drug can be absorbed through passive diffusion. iii. There is no use of energy. iv. Always from higher concentration to lower concentration. v. Driving force is concentration gradient. vi. Governed by several factors: 1. Exposed surface area- more surface area more absorption, less surface area, less absorption. This is what makes the intestines a better site for absorption than the stomach. 2. Gradient- if it is high then the absorption is faster. 3. Solubility (chemical nature)-more lipophilic or less polar are more likely to be easily passively transported. d. Active transport requires energy and is against the gradient. i. Carrier mediated- involves a protein or a carrier 1. Glucose is water soluble and can’t be transported passively. 2. It requires a transporter, like with food. e. Facilitated requires carrier, but is passive- does not require energy vii. Many mechanisms by which drugs are transported from GIT to systemic circulation viii. Other factors blood flood, surface area, and contact time VII. Factors affecting drug distribution [S7] a. Blood flow b. Capillary permeability i. For example the capillary permeability is different in the brain and the liver. c. Binding to proteins i. Once it gets absorbed, the bioavailability also depends on the binding property to protein. ii. Albumin is most abundant protein in blood. iii. Many drugs, mostly acidic drugs, bind to albumin reversible. iv. A few drugs bind covalently, but mostly reversibly v. Only unbound or free drug is available for bioactivity. d. These are some of the limiting factors effecting drug absorption and distribution. VIII. What are pH and pK? [S8] a. What is pH? pH is - log of H+ concentration b. The role of pH in drug absorption i. There are several drugs that are weak acids. Mostly drugs are a weak acid or weak base ii. If you know the pH of the GI tract and the nature of a compound, you can estimate the absorption of a drug FUN2: 11:00-12:00 Scribe: Teresa Kilborn Monday, December 1, 2008 Proof: Joan-Marie Manolakis Dr. Prasain Pharmacology Page 3 of 4 iii. Drugs have different pKa values 1. pKa measures the propensity to give or accept the hydrogen concentration iv. In an equation, pH is actually pKa when 50% of the weak acid is ionized (the log will be 0, thus pH = pKa) v. pKa is an important characteristic of a drug that determines where it gets absorbed. vi. Stomach and intestine have different pH’s, and only unionized molecules get absorbed. vii. Thus, we need to find an environment where weak acid or base can exist in the unionized form. viii. The role of pH in drug absorption [S9]- the stomach is very acidic with a pH < 3. 1. Think about a drug like aspirin. a. What will be the fate in the stomach? In an acidic environment, an acidic compound does not tend to dissociate. In order to dissociate a weak acid you need a higher pH that will facilitate movement in the right direction, more unprotonated species. In the stomach, the acidic environment causes the acidic drug to exist mostly in the unionized form. b. The other extreme is plasma. The plasma has neutral or basic pH. A weak acid in the plasma would undergo ionization and make more unprotonated species. In the stomach, an acidic drug will get absorbed and once absorbed and in the plasma, the environment is neutral or slightly basic causing the compound to exist in unprotonated form. Ionic form is not good for absorption. c. Acidic drugs do not ionize in the stomach. It is absorbed. ix. What about weak basic drugs- just opposite. The absorption of a weak acid or base depends on the environment- which form is more predominant, the ionized or unionized form. x. Aspirin [S10]1. pH= pKa – log (ionized/unionized) 2. Aspirin has a pKa of 3.4 and the stomach has a pH 1.4. 3. Take the log of – 2. 4. Unionized form of aspirin is 100-fold greater than the ionized form, so that indicates that the absorption of aspirin is favored. c. Problem? [S11] i. READ OFF SLIDE ii. When you take antacid, it raises the pH. When you raise the pH, then the aspirin will ionize in your stomach and you can no longer absorb it efficiently. IX. Bioavailability [S12] a. The issue of absorption is directly related to bioavailability b. When you inject a drug (IV), the total amount of drug is in circulation. So, the bioavailability is very good. c. Bioavailability is the fraction of unchanged drug that reaches the systemic circulation. d. When you inject the drug IV, it is 100 % bioavailability. When you take the drug orally, there is a question of how much really gets to your blood. It is always less than 100%. e. The bioavailability is the area under the curve (AUC) during oral administration (O) divided by the area under the curve (AUC) during IV injection of the same drug f. Bioavailability=(AUC) O/ (AUC) IV g. The action of metabolism will determine the bioavailability. X. Protein binding [S13] a. There are several drugs that when you want to calculate the free drug amount in your plasma concentration, you have to think about the protein binding. b. Several proteins bind to the drug. c. Most importantly albumin, especially in weak acid drugs and glycoproteins in weak base drugs. d. Unbound drug is biologically active. e. Protein binding to a drug is important in bioavailability. f. Bioavailability means the unchanged drug in the plasma concentration. XI. Phase I and phase II metabolism [S14] a. Drugs are chemical, have biological activity, and must be excreted. b. For that reason, once inside it undergoes metabolism. c. Why should drugs be metabolized? Metabolism means inactivation. Most drug like molecules are lipophilic, and more soluble for passive diffusion. When you have to excrete them, lipophilic characteristic is not good. There is a mechanism by which you convert the molecule to be more hydrophilic so you can excrete it in urine or feces. There is phase I and phase II metabolism. d. Metabolism starts in the GIT (intestinal epithelium) FUN2: 11:00-12:00 Scribe: Teresa Kilborn Monday, December 1, 2008 Proof: Joan-Marie Manolakis Dr. Prasain Pharmacology Page 4 of 4 e. The liver is the major organ of metabolism. f. Phase I metabolism is functionalization-it introduces a functional group or hydrolyzes or oxidizes. g. Phase II is conjugation reactions like sulfation, gluconation, glucuronidation. Making the drug more hydrophilic so it helps to inactivate and excrete. i. Exception is morphine: 6-gluconated morphine is more potent than the parent compound, morphine. ii. Most of the time gluconation or sulfonation inactivates and helps with excretion in urine. He will continue with drug distribution and elimination tomorrow.