* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death
Remote ischemic conditioning wikipedia , lookup
Coronary artery disease wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Cardiac surgery wikipedia , lookup
Pericardial heart valves wikipedia , lookup
Management of acute coronary syndrome wikipedia , lookup
Myocardial infarction wikipedia , lookup
Electrocardiography wikipedia , lookup
Artificial heart valve wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Ventricular fibrillation wikipedia , lookup
Heart arrhythmia wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
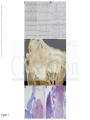
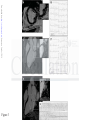
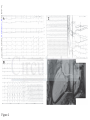
DOI: 10.1161/CIRCULATIONAHA.115.016291 Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death Running title: Basso et al.; Arrhythmic Mitral Valve Prolapse Cristina Basso, MD, PhD1*; Martina Perazzolo Marra, MD, PhD1*; Stefania Rizzo, MD, PhD1; Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Manuel De Lazzari, MD, PhD1; Benedetta Giorgi, MD2; Alberto Cipriani, MD1; Anna Chiara Frigo, g MSc1; Ilaria Rigato, g MD, PhD1; Federico Migliore, g MD, PhD1; Kalliopi p Pilichou, PhD1; Emanuele Bertaglia, MD1; Luisa Cacciavillani, MD, PhD1; Barbara Bauce, M MD, D, P PhD hD1; Domenico Corrado, MD, PhD1; Gaetano Thiene, MD1; Sabino Iliceto, MD1 1 Dept Dep De pt of Ca Cardiac, Thoracic, and Vascular Scienc Sciences; cess; 2Dept of Ra Radiology, adi d ology, Azienda OspedalieraUniv versity of Padua Padua Medical Pa Mediicaal School, Scho Sc hoool, Padua, ho Padu dua, Italy du Itaaly y University *contributed *con *c ontr on trib tr ibut ib uted ut ed eequally qual qu ally al ly Address for Correspondence: Cristina Basso, MD, PhD Department of Cardiac, Thoracic, and Vascular Sciences University of Padua Medical School Via A. Gabelli, 61 35121 Padova-Italy Tel: +39 0498272286 Fax: +39 0498272284 Email: [email protected] Journal Subject Codes: Diagnostic testing:[30] CT and MRI, Etiology:[5] Arrhythmias, clinical electrophysiology, drugs 1 DOI: 10.1161/CIRCULATIONAHA.115.016291 Abstract Background—Mitral valve prolapse (MVP) may present with ventricular arrhythmias and sudden cardiac death (SCD) even in the absence of hemodynamic impairment. The structural basis of ventricular electrical instability remains elusive. Methods and Results—A) The Cardiac Registry of 650 young adults (40 yrs) with SCD was reviewed and cases with MVP as the only cause of SCD were reexamined. Forty-three MVP Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 cases (26 female, age range 19-40, median 32 yrs) were identified (7% of all SCD, 13% of women). Among 12 with available ECG, 10 (83%) had inverted T waves on inferior leads and all right found ight bundle branch block ventricular arrhythmias. A bileaflet involvement was fo oun undd in 770%. 0%. 0% LV fibrosis was detected at histology at the level of papillary muscles in all and infero-basal wal wall in 88%. MVP ventricular n 88% 8%.. B) M 8% VP P ppatients atients with complex ventricu ula larr arrhythmias (N=30) 0)) aand nd without (controls, N=14) underwent study protocol cardiac magnetic N=114) underwen ent a st en stud udyy pr ud prot otoocol ot ocol o iincluding nclu nc luding ng g ccontrast-enhanced ontra rast-eenh ra nhan ancced an ced ca card rdia rd iacc m ia agn gnet gn etic et ic rresonance eson es onnan ance ce ((CECECE CMR). Patients with arrhythmias (22 age 28-43, median CM MR) R). Patien ents w en ith ccomplex om mpleex ve vventricular nttri ricu ulaar ar rrh rhyt ythm mias (2 22 ffemale, ema male ma lee, ag ge rrange anngee 28 28-43 3, m 3, ed diaan 41 yrs), either branch polymorphic, yrrs) yrs) s), ei eith ther th er rright ight ig ht bbundle un undl ndl dlee br bran anch an ch bblock-type loc ockk-ty type pe oorr po poly lymo morp mo rphi rp hicc, sshowed hi howed ho ed a bbileaflet ilea il eafl ea flet fl et iinvolvement nvol nv olve ol veme eme ment nt iin n 70% of cases. LV late-enhancement was identified by CE-CMR in 93% vs. 14% of controls (p<0.001), with a regional distribution overlapping the histopathology findings. Conclusions—MVP is an under-estimated cause of arrhythmic SCD, mostly in young adult women. Fibrosis of papillary muscles and infero-basal LV wall, suggesting a myocardial stretch by the prolapsing leaflet, is the structural hallmark and correlates with ventricular arrhythmias origin. CE-CMR may help to identify this concealed substrate for risk stratification. Key words: arrhythmia (mechanisms), mitral valve, sudden cardiac death, arrhythmia, pathology, cardiac magnetic resonance imaging, mitral valve prolapse 2 DOI: 10.1161/CIRCULATIONAHA.115.016291 Introduction Mitral valve prolapse (MVP) is the most common valve disease with an estimated prevalence of 2-3% in the general population1. Although MVP is generally regarded as a benign condition2,3, the outcome is widely heterogeneous and complications such as mitral regurgitation, atrial fibrillation, congestive heart failure, endocarditis and stroke are well known. Ventricular arrhythmias and sudden cardiac death (SCD) have been even reported4-7. From a pathologic anatomy viewpoint, accumulation of proteoglycans (myxomatous Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 mitral valve) is the most common cause of MVP, accounting for leaflet thickening and redundancy, chordal elongation, interchordal hoodings and anular dilatation8. While these valve abnormalities well explain mitral regurgitation and mechanical complications duee tto o en enha enhanced hanc ha nced nc ed extensibility, the pathogenesis of ventricular arrhythmias/SCD in MVP remains controversial. The The es eestimated tima mate ma t d rate of SCD in MVP range ranges es fr from 0.2% to 0.4% per per year on the basis of studies pprospective rosspective fo ffollow-up lllow-u ow up stud st tud udiies ies4. Le Left ft ven ventricular enttricul u ar (LV (LV) LV V) dy dysf dysfunction sfun sf fun uncctio ionn du duee to sev severe ver eree mi mitr mitral tral tr al regurgitation egu gurg rgitatio rg on identifies i en id e tifiees a patient pattie i nt nt subgroup suubg b rooup up at at high high g risk riisk off SCD CD9. Ho Howe However, w verr, lif life-threatening ifeif e--threeat ateenin ng veent vent ntri ricu ri cula larr ar la arrh rhyt rh ythm thm hmia iass oc ia occu curr al also so iin n MV MVP P pa pati tien ti entts with en wiith ttrivial rivi ri vial ial oorr ab abse sent se nt m itra it rall re ra regu gurg rgit rg itat it atio at ionn10. io ventricular arrhythmias occur patients absent mitral regurgitation Previous pathology studies of SCD mostly focused on the mitral valve or conduction system abnormalities as cause of electrical instability8,11-17, while the demonstration of a myocardial source of arrhythmias remained elusive18-20. The aim of our report is to demonstrate that MVP is a significant cause of SCD and lifethreatening arrhythmias in young adults due to an underlying myocardial substrate, which is detectable by contrast-enhanced cardiac magnetic resonance (CE-CMR) and may serve for risk stratification and SCD prevention. 3 DOI: 10.1161/CIRCULATIONAHA.115.016291 Material and Methods Study populations A) SCD victims with MVP In the time interval 1982-December 2013, all the hearts of SCD victims 40 years old occurring in the Veneto Region, North East Italy (geographic area 18,368 km2, overall population 4.857.210 according to the Italian Census Bureau 2011), were collected, pathologically investigated and preserved. SCD is herein defined as witnessed sudden and unexpected death occurring within 1 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 hour of the onset of symptoms or death of an individual who had been seen in stable condition <24 hours before being found dead21,22. Demographic, clinical and pathologic data were recorded in the electronic database of the Registry of Cardio-cerebro-vascular pathology which act acts ts as rreferral efer ef erra er rall ra center for SCD of the North-East of Italy. Charts Char Ch arts ar ts were weere ere evaluated for age, sex, sympto symptoms toms to m and clinical history history. y. Al A Alll the hearts were reexamined carefully standardized eex xamined car ref efullly y aaccording cccor ordding ng tto o a st sta anddard rdizzed pprotocol rd ro otoco coll21. SCD co SCD ca case cases sess w were ere selected sel elec eccte tedd inn whom who hom m MVP MV P due to om myxomatous yx xom mattou ous valve vaalv l e disease dise seaasee was se was the th he only onnly y cardiac caarddiacc abnormality abn norma mali ma l ty y ffound ou und at au auto autopsy. topssy.. to Myxomatous M My yxo xoma oma mato tous to uss val valve alve al vee ddisease isea is ease ea se is is defined defi de fine fi nedd as increased ne inc ncre reas re aseed le as leaf leaflet afle af lett le le leng length ngth ng th and and redundancy, red edun und ndan ancy cy, with wiith interchordal int nter erch er chor ch orda or dall da hoodings and leaflet billowing toward the left atrium and chordae tendineae elongation8. In the absence of extracardiac (cerebral, respiratory) or mechanical cardiovascular explanations, the cause of death was considered cardiac arrhythmic. Exclusion criteria were clinical and/or pathologic evidence of more than mild mitral regurgitation. Hearts from 15 sex and age-matched patients (10 females, mean age 30 years, range 18-40), who died suddenly for extracardiac causes (8 cerebral and 7 respiratory), served as controls. B) MVP patients with complex ventricular arrhythmias The study included consecutive patients, referred to the Cardiology Clinic, from January 2010 to 4 DOI: 10.1161/CIRCULATIONAHA.115.016291 December 2013, with complex ventricular arrhythmias detected on the basis of 12-lead 24-hour Holter monitoring and echocardiographic diagnosis of MVP, defined as >5 mm thickening and >2-mm displacement of one or both mitral leaflets into the left atrium as viewed in the LV outflow tract orientation23. Twelve-lead ECG 24 hours Holter was requested due to the presence of either arrhythmic symptoms or 12-lead ECG changes. Complex ventricular arrhythmias consisted of ventricular fibrillation (VF) and ventricular tachycardia (VT), either non-sustained or sustained24. Complex ventricular arrhythmias patients were further sub-divided into two Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 groups, i.e. those with 3 ventricular premature beats (VPB) run and those >3 VPB run. The control group consisted of MVP patients with minor ventricular arrhythmias, i.e. solated VPB, couplets and bigeminal VPB. isolated Exclusion criteria were significant mitral regurgitation, tricuspid dysplasia or eguurg rgit itat it atio at ion, io n, car ardi ar d omyopathies orr congenital he di ear artt abnormalities, hem mod odynamic unstable regurgitation, cardiomyopathies heart hemodynamic conditions con nd and ccontraindication oon ntrraind ndic dic icaatio ionn to C io MR. T MR he study yw as app ppro pp r ve ro vedd by tthe he ins he sti titu tuti tu tion ti onal on al rreview evie ev iew ie w conditions CMR. The was approved institutional booar ard, d and aall ll pa atieentss gave ga iinformed nffor o me medd co cons n en nt.. board, patients consent. Prot Pr otoc ot ocol oc olss of iinvestigation. ol nves nv esti es tiga ti gati ga tion ti on. on Protocols A) Pathologic anatomy study Formalin-fixed hearts were restudied according to a protocol previously reported21. Leaflet involvement (whether anterior, posterior or bileaflet) and the presence of endocardial fibrous plaque (friction lesion) on the LV infero-basal wall were assessed. Multiple samples of the LV and right ventricular free walls and septum, including the papillary muscles (PMs), were obtained for histology. Additional samples were taken in the LV infero-basal free wall, underneath the posterior mitral leaflet. Five μm-thick sections were stained with HematoxylinEosin, Weigert-van Gieson, Heidenhain trichrome and Alcian-PAS. Morphometric analysis was 5 DOI: 10.1161/CIRCULATIONAHA.115.016291 performed using an Image-Pro Plus program (Version 4.0. Media Cybernetics, MD, USA) to quantify the fibrous tissue percent area of LV myocardium on Heidenhain trichrome stained sections at 25x magnification. Mean cardiomyocytes diameter was calculated on Haematoxylineosin stained sections at 400x magnification. Quantitative analysis was performed by two blind expert pathologists (CB, SR) with an interobserver variability <5%. B) Clinical study All patients underwent cardiovascular evaluation including history, physical examination, 12Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 lead electrocardiogram (ECG), 2D-transthoracic echocardiography, 12-lead 24-hour Holter monitoring and CE-CMR. Coronary angiography was performed in selected cases. The 12-lead revi re viou vi ousl ou slyy sl ECG at rest and the 24-hour Holter monitoring were independently assessed as pre previously eported25 by two experienced observers (MDL and DC) who were blinded to patient data. reported NonNo n-su ns sttai ainned VT was defined as 3 con onnsec secutive VPBs with a ra ate > 100 bpm that lasted Non-sustained consecutive rate < <30 300 seconds du during uri rinng 224-hour 4-ho 4hour ho ur H Holter olte ol ter mo te mon monitoring. nitori ring ri g. Su Sustained ustaine nedd VT w ne was as ddefined efin ef ineed aass tachycardia in tach ta chyc ch ycarrdia yc dia originating orrig igin inatingg in thee ventricle in venttricle with witth rate ratte >100 >1 100 bbeats/minute eats ea t /m min nutte an aand d la lasting astiing > >30 30 0 seco seconds ondds orr re requ requiring uiriing an an intervention nte terv rven enti en tion ti on ffor or ttermination. ermi er mina mi nati na tion ti on. on Cardiac magnetic resonance was performed on a 1.5-Tesla scanner (Magnetom Avanto, Siemens Medical Solutions, Erlangen, Germany). All patients underwent detailed CE-CMR study protocol as previously described25. The presence and location of late gadolinium enhancement (LGE) were independently assessed by two experienced observers (MPM and BG) who were blinded to clinical data. To exclude artifact, LGE was deemed present only if visible in two orthogonal views (long-axis and short-axis). LGE was identified using a signal intensity threshold of >5SD above a remote reference region and quantified according to a previously reported method. 6 DOI: 10.1161/CIRCULATIONAHA.115.016291 Statistical Analysis Data are expressed as mean value±standard deviation or median with 25 to 75 percentiles for normally distributed and skewed variables, respectively. Normal distribution was assessed using Shapiro-Wilk test. Categorical differences between groups were evaluated by the chi-square test or the Fisher exact test as appropriate. Paired and unpaired t test were used to compare normally distributed continuous variables respectively obtained from the same patient and different patients; Wilcoxon signed rank test (same patient) and Wilcoxon rank sum test (independent Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 samples) were used for skewed continuous variables. A p value <0.05 was considered significant. The minimal detectable effect at a significance ignificance level of 5% and power at 80% is equal to 1 (with non parametric test) t) ffor or quantitative variables (Cohen’ Effect); for binary data an odds ratio of at least 10 can be detected iff the complex hee proportion pro ropo port po rtionn of the characteristic test is equal rt al tto o 10% in the no com mpl x ventricular mple arrhythmias arrh ar hythmias group grou oupp (Fisher’s ou (F Fishe ishe her’ r s exact exaact ex act test).Statistics test te stt). ) Stattis i tiics were we analyzed analy naly lyzzedd with with SPSS SPS PSS version vers ve rsio rs ionn 19 io 1 (SPSS (SP SPSS SS Inc, Inc Chicago, IL). Ch hiccag a o, IL) L). L) Results A) SCD victims with MVP Among 650 consecutive young SCDs recorded in the Veneto Region Registry , 43 cases (26 females, median age 32 years, range 19-40) with MVP due to myxomatous valve disease were identified. They represent 7% of all SCD cases and 13% of women who died suddenly, being the first structural cause in the latter group. Main clinical and pathologic data are reported in Table 1. SCD occurred mostly at rest or during sleep (N=35, 81%). Twenty (47%) had an in vivo diagnosis of MVP, with auscultatory click in 18 (90%) and palpitations in 14 (70%). Nine (21%) 7 DOI: 10.1161/CIRCULATIONAHA.115.016291 were under beta-blockers therapy due to non sustained ventricular arrhythmias. ECG was available in 12 (28%), showing negative/isodiphasic T waves on inferior lead in 10/12 (83%) (Figure 1A,B); all had right bundle branch block (RBBB) morphology (100%) ventricular arrhythmias. In SCD cases with MVP, valve leaflets were redundant, thick and elongated, with either isolated posterior (N=13, 30%) or bileaflet (N=30, 70%) involvement (Figure 1C). The involvement of the posterior leaflet was diffuse in 23 (53%) and confined to the medial scallop in Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 20 (47%). Endocardial fibrous plaques in the postero-lateral wall were found in 25 (58%). Microscopic examination of the LV myocardium showed an increased endo-perimysial and patchy replacement-type fibrosis at the level of PMs and adjacent free wall in n aall ll ((Figure Figu Fi gure gu re 1D,E and Figure 2). Similar findings, with a subendocardial-midmural layer distribution, were detected infero-basal leaflet, deteect cted ed iin n th the in nfe fero-basal wall, underneath thee pposterior o terior mitral valve le os leaf a let, in 38 cases (88%). The Thee mean fibrous fib bro ous tissue tisssu suee percent perc pe rcen rc entt area en area in in MVP MV VP SCD D victims vict ctim ct imss was im wass 30.5% 30.5% 5% att thee level level evel of of PMs PMs and and 33.1% wall myocardium (vs. and controls, p<0.001). 333.1 .1% % in the hee iinfero-basal nffero-baasaal wa alll m yoca yo c rd diu ium m (v vs. 6.3% an nd 66.4% .4% 4% iin n co onttro ols, p< p<0.00 01). 1) In n tthe he same ame areas, are reas as, the as the cardiomyocytes card ca rdio rd iomy io myoc ocyt oc ytes tes showed sho howe wed ed increased incr in crea cr ease ea sedd diameter se diam di amet am eter et er (19.2±6.0 (19.2±6 2±6.00 micron mic icro ronn vs. ro vss 12.8±0.4, 12.8±0 8±0.4, 4 p<0.001) and dysmorphic and dysmetric nuclei. B) MVP patients with complex ventricular arrhythmias The baseline clinical and CMR findings are summarized in Table 2. Fourteen MVP patients with or without minor ventricular arrhythmias (i.e. isolated VPB, couplets and bigeminal VPB) served as controls. Thirty MVP patients (22 female, median age 41) with complex ventricular arrhythmias, i.e. 1 VF (N=2, who had also non-sustained VT) and VT (N=28) - either non-sustained (N=27) or sustained (N=1)- were collected. VT of LV origin (RBBB morphology) was present in all, 8 DOI: 10.1161/CIRCULATIONAHA.115.016291 with either inferior (43%) or superior (87%) axis. Among the 27 patients with non-sustained VT, the mean length was 4 beats (ranging 3-11 beats). Complex ventricular arrhythmias occurred at rest in 26/30 (87%). All patients had normal QTc (mean 423, range 409-440). Exercise stress test, performed in 20,was negative for effort-induced ventricular arrhythmias. Bileaflet MVP was present in 21 (70%) patients with complex ventricular arrhythmias vs. 5 (36%) controls (p=0.031). On post-contrast sequences, LV-LGE was identified in 28 (93%) vs. 2 (14%) (p<0.001). Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 By dividing the MVP population with complex ventricular arrhythmias into two sub-groups, 20 patients had 3 VPB run and 10 patients >3 VPB run (p>0.05). In MVP patients with complex comparing those ventricular arrhythmias, no difference was found in terms of LV-LGE when comp mp par arin ingg th in thos osee os with 3 VPB run and those >3 VPB run (p>0.05). The mid-apical The LGE L E was LG was localized on the PMs in 255 ppatients a ients (83%), with a mi at m d-apical distribution in segment, underneath 1166 and/or and/or basall adjacent adjjace ad cent ntt free fre reee wall w ll in wa in 24 cases; casees;; andd on on the the LV LV infero-basal in nfe f ro-b -bas bas asaal seg egm eg ment ment nt, un unde dern de rnea rn eath ea th the 3A-D). focal LGE region, he posterior posterio po or leaflet, l affleet, inn 22 le 22 (73%) (73 73%) 73 %) (Figure (Fi Figu uree 3A A-D D). A fo ocaal eendocardial ndo doca do caardia rdiaal LG L GE in the ssame am re ame egionn, featuring fibrous plaque, found patients feat fe atur at urin rin ingg a fi fibr brou br ouss pl plaq aque aq ue, e was waas fo foun und nd in 112 2 pa pati tien ti ents en ts ((40%). 40%) 40 %). %) The median LV LGE % was 1.2 in MVP with complex ventricular arrhythmias vs. 0 in MVP without (p<0.01). Two MVP patients experienced aborted SCD due to VF, despite beta-blocker therapy due to previous sustained VT. Detailed invasive and non-invasive evaluation ruled out cardiac causes other than MVP. Both had RBBB pattern ventricular arrhythmias with superior axis and T waves abnormalities on inferior leads. CE-CMR, performed 6 and 10 months before aborted SCD, revealed LV LGE of PMs and infero-basal wall (Figure 3 E,F). Both patients received an implantable cardioverter defibrillator (ICD). 9 DOI: 10.1161/CIRCULATIONAHA.115.016291 One patient had pre-syncopal episodes despite bisoprolol therapy. She underwent electrophysiological study with induction of sustained VT with the same RBBB morphology of VPBs (Figure 4A-C). The CE-CMR showed a non-ischemic LGE pattern in the LV infero-basal wall (Figure 4D). She also underwent ICD implantation. Of the three patients with ICD (mean follow-up 10 months), two had non sustained-VT: one patient with spontaneous interruption and the other requiring anti-tachycardia pacing. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Discussion MVP is an under-recognized cause of SCD in young adults, accounting for 7% of total fatal with events and 13% of female victims in our large Cardiac Registry experience. The ppatient atie at ient ie nt w ithh it MVP and ventricular arrhythmias at risk of SCD is usually a young adult woman, with a midsystolic auscultation, involvement ysttol olic ic cclick lick li ck at au ausc s ultation, bileaflet involvem men entt of the mitral valve, T wave abnormalities on inferior RBBB-type polymorphic arrhythmias ECG. Clear-cut nfeerior leads, RB RBBB BB B-typ ypee or po yp poly lym ly morp morp rphhic vventricular enntriccullar ar arrh rhyt rh ythm yt hmia hm i s on E ia CG. Cl Clea earea r cu rcutt ev eevidence iden id encee en electrical instability herein provided first consists off a ssubstrate ubstraate off el lectrriccal ins nstaabi ns b liity inn MV MVP P iiss he ereeinn pr prov vid ded d ffor or tthe or h fi he irstt ttime im me an andd co onssistss ooff myocardial LV wall, underneath myoc my ocar oc ardi ar dial di al scarring sca carr rrin rr ingg targeting in targ ta rget rg etin et ingg the in the PMs PMs and and the the infero-basal infe in fero fe ro-bas bas asal al L V fr free ee wal alll, und al nder nd erne er neat ne athh th at thee posterior leaflet, well in keeping with the site of origin of RBBB-type ventricular arrhythmias. The LV myocardial fibrosis observed at histology in SCD victims was then confirmed in the clinical arm of the study, with evidence of LGE at CE-CMR in arrhythmic MVP patients, thus pointing to a promising role of this non invasive technique for risk stratification beyond traditional prognostic markers. MVP: an underappreciated cause of SCD The absence of uniform diagnostic criteria of MVP in the general and forensic pathology practice and the frequent consideration of this valve disease as an uncertain cause of SCD are major 10 DOI: 10.1161/CIRCULATIONAHA.115.016291 obstacles to provide data on the real burden of MVP based upon a metanalysis of published studies21. With these shortcomings, the prevalence of MVP in pathology series of SCD in the young ranges from 0 to 24%20,22,26,27. By adopting strict criteria for definition of myxomatous mitral valve, in the Veneto Region SCD Registry MVP accounted for 7% of all cases in young adults (<40 years) and 13% among women, representing the first structural cause in the latter group. The diagnosis can be easily established at macroscopic examination and then confirmed by routine histology, but it might be overlooked by superficial inspection, leading to discharge Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 the heart as normal. Our data are likely to change the current thinking about MVP as a benign condition and point to the need to draw the attention of forensic pathologists to an entity that has been largely underestimated so far. Ventricular arrhythmias in MVP nM VP sseries erie er ies wi ie with th prolonged ECG recording, a vvariable ar ariable prevalence off vventricular entricular arrhythmias In MVP ha been reported, has reporte ted, te d, reflecting ref efllect ef lect ctin ingg the in the different diff di fferren ff nt MVP MV definition, deefi finiti tion ti on, the on the population popu po pula pu lattio la ionn studied stud udie ud iedd and ie an the th 5,9,10,28-35 5,9,10,2 ,28 28-3 -35 -3 co omp mplexity y ooff ve enttricu ulaar ar rrh rhyt ythm yt mia ias co onsid ideered5,9 id . In pparticular, arrtiicu ula l r, tthe hee cl clinical lin i icaal eevidence vide deencee ooff complexity ventricular arrhythmias considered hemo he mody mo dyna nami na mica mi call ca lly im ll impo port po rtan rt antt re an regu gurg rgit rg itat it atio at ionn gr io grea eatl ea tly iimpacts tl mpac mp acts ac ts oon n th thee oc occu curr rren rr ence en ce ooff vent ve ent ntri ricu ri cula larr la hemodynamically important regurgitation greatly occurrence ventricular arrhythmias. However, the detection of MVP in survivors of life-threatening arrhythmias suggests that a true association between hemodynamically uncomplicated MVP and arrhythmic SCD may exist9. Thus, we decided to focus on "pure" MVP, excluding MVP associated with valve incompetence and LV remodelling, not to defile the message by over-reporting ventricular arrhythmias. Early electrophysiological studies demonstrated that the most common site of origin of VPB is the infero-basal portion of the LV36. In the recent study of malignant MVP by Sriram et al37, frequent VPBs originated from the outflow tract and PMs. Moreover, electrophysiology mapped the site of origin to the PMs, the LV outflow tract and the mitral annulus, as to suggest 11 DOI: 10.1161/CIRCULATIONAHA.115.016291 that VPBs arising close to the prolapsing leaflet and adjacent structures are the arrhythmic triggers. From a pathophysiologic perspective, the mechanism of ventricular arrhythmias in MVP patients with trivial or absent mitral regurgitation remains speculative9,38. MVP-related factors have been first advocated, such as the excessive traction on the PMs by the prolapsing leaflets39; the mechanical stimulation of the endocardium by the elongated chordae, with after depolarization-induced triggered activity; the diastolic depolarisation of muscle fibres in Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 redundant leaflets with triggered repetitive automaticity40; and the endocardial friction lesions with extension into the myocardium41. Moreover, the coexistence of extravalvular diseases has been suggested, including autonomic nervous system dysfunction42, conduction ssystem yste ys tem te m abnormalities13, fibromuscular dysplasia of small coronary arteries19 and occult 10,43 1 10 43 card dio iomy myop my opat op athiees10, at . cardiomyopathies T The hee myocardial al ssubstrate ub bstra rate ra te ooff el elec electrical ectr ec tric tr icall ins ic instability sta abiliity y in n MV MVP P Previous pathology studies MVP patients mostly Pr rev evio i us pat io attho h lo ogy y stu udies iin nM VP P pa atieentss ddying y ngg ssuddenly yi udddeenlyy mo mos stly y ffocused occussed d onn mitral mittraal valve vaalvve structural tru ruct ctur ct ural ral aalterations, lter lt erat er atio at ions io ns, su ns ssuggesting ugg gges gg esti es ting ti ng a ro role le ffor or aannular nnul nn ular lar ccircumference, ircu ir cumf mfer mf eren er ence en ce, le ce leaf leaflets afle af lets le ts llength engt en gthh an gt andd th thic thickness ickn ic knes kn esss es and presence and extent of endocardial plaques8,14-17. Surprisingly, no investigation did systematically address the LV myocardium to search for the substrate of electrical instability, except for few anecdotic cases11,13,14,44,45. For the first time, we extended the histopathology investigation beyond the valve in all SCD cases and provided convincing evidence of fibrosis in the LV myocardium, which is closely linked to the mitral valve, i.e. the PMs with adjacent free wall and the infero-basal wall. The LV myocardial scarring is qualitatively different from that observed in ischemic heart disease, where it is usually compact and confluent, being instead patchy and interspersed within surviving, hypertrophic cardiomyocytes. Noteworthy, previous 12 DOI: 10.1161/CIRCULATIONAHA.115.016291 pathology studies addressed the so-called “idiopathic myocardial fibrosis” in SCD victims46. By definition this entity is not associated with other structural heart diseases and remains without an explanation. However, the LV fibrosis described in our MVP cases differ in terms of type (i.e. scarring) and location (i.e. LV papillary muscles and basal postero-lateral wall). Furthermore, we herein demonstrate that CE-CMR can detect LV-LGE in MVP patients with complex ventricular arrhythmias, closely overlapping the histopathologic features observed in SCD victims. At the level of PMs, two LGE sites have been found, i.e. the mid-apical portion Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 and the base/adjacent LV wall. Although PMs LGE has been reported by Han et al24 in MVP patients with a history of arrhythmias, most of these patients had moderate to severe mitral regurgitation. egurgitation. While confirming f these data in purely arrhythmic MVP patients wi without ith thou outt ou hemodynamic impairment, we first provide convincing evidence of LGE in the infero-basal LV wall. wall l. The The arrhythmogenic arrh ar rhythm rh hmogenic role of the LV myocardial hm myocaard rdia ial scarring is suppor ia supported rte tedd by the morphology of arrhythmias arrh ar hythmias andd byy eelectrophysiological lect le ctro ct roph ro phys ph ysiiolo ys iolo logi giccall studies gi sttud udiees inn MVP MVP indicating ind ndic icat ic atin i g that that the thhe most most ost common comm co mmon mm on site sit itee of o 36,37 6,37 VPB LV wall VP B origin is is the th he infero-basal infeero ro-bassal L V wa w lll36 . Most Mo st ooff CE -CMR CMR sstudies tudi tu dies di es ffor or aarrhythmic rrhy rr hyth thmi th micc ri mi risk sk sstratification trat tr atif at ific if icat ic atio at ionn ar io aree co comi ming mi ng ffrom rom ro m ei eith ther th er CE-CMR coming either ischemic heart disease or cardiomyopathies, with the notion the larger the LGE burden the worse the prognosis. Our quantitative data suggest that the volume of LV scarred tissue in MVP is relatively small but still associated with SCD.. We should recognize that MVP differs from other non-valvular diseases in terms of LGE distribution (“stretched areas”) and amount. Furthermore, the mechanical stretch by the prolapsing leaflet and elongated chordae could act as a trigger of electrical instability. Further studies with higher number of MVP patients are needed to confirm these preliminary data of LV LGE. Since the early descriptions, abnormal LV contraction pattern and ECG abnormalities 13 DOI: 10.1161/CIRCULATIONAHA.115.016291 suggested that MVP has a significant myocardial involvement47-51. The hypothesis that the socalled “MVP syndrome” is a cardiomyopathy, where regional hypercontractility acts as the primum movens of mitral valve geometry disruption, with abnormal tension on the chordae and leaflets and secondary increase in myxomatous tissue and leaflet thickening, has been even advanced50,51. Our pathology and CE-CMR data support the theory that LV abnormalities are rather the consequence of MVP, due to a systolic mechanical stretch of the myocardium closely linked to the valve, i.e. PMs and infero-basal wall, by the prolapsing leaflets and elongated Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 chordae, accounting for a localized hyper-contractility, with myocyte hypertrophy and injury leading eventually to fibrous tissue repair. The increased cardiomyocyte diameter, in the same areas showing replacement-type fibrosis, is in keeping with this theory. Considering that performance of CE-CMR in all MVP patients would be an expensive prop pos osit itio it ion, io n, some som me clinical c inical markers that could target cl targ rget rg et a high-risk subgroup subgrouup destined for screening proposition, by CE-CMR by CE-CMR are arre needed. need eded ed ed.. ECG ed ECG depolarization depol epol olar arizzattio ar ionn abnormalities abno norrmal alit al itie it iess on infero-lateral ie inffer eroo latteraal leads, olead le ads, ad s, complex com ompl plex pl ex ve ent ntri r cular ar ri arrh r ythmiaas (( 3 VPB VPB P run) run u ) with with h RBBB RBBB B morphology morpphoology mo logy on on 12-lead 122-lleaad ECG EC Holter Hollter Ho ventricular arrhythmias moni mo nito ni tori to ring ri ng and and a hhistory isto is tory to ry ooff pr pre e-sy sync ncop nc opee, syncope op sync yn ncop opee an andd ab abor orte or tedd SC te SCD D se seem em tto o re repr pres pr esen es entt an en monitoring pre-syncope, aborted represent indication for CE-CMR. Finally, we recognize that our data support an association between anatomic substrate and risk, in an entity that is underappreciated as a cause of SCD and also has a low enough incidence that any marker of increased risk might be of significant value to the clinician. Beta-blockers are commonly used to treat arrhythmias in MVP patients. The fact that 21% of young adult SCD victims and two living patients had aborted SCD despite beta-blocker therapy is disappointing but not surprising10. Prospective multicenter studies are warranted to support the role of CE-CMR and electrovoltage mapping for risk stratification and to assess the 14 DOI: 10.1161/CIRCULATIONAHA.115.016291 efficacy of antiarrhythmic therapy and targeted catheter ablation in selected cases. Limitations of the study While acknowledging the small number of MVP patients without complex ventricular arrhythmias, we should recognize that it is difficult to collect “pure” MVP patients without either valve incompetence or ventricular arrhythmias both clinically and at postmortem. Prospective multicenter studies enrolling a higher number of MVP, with and without complex ventricular arrhythmias, are warranted to evaluate the exact prevalence of LGE in the overall MVP Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 population. Genetic data are not available in our SCD population. Noteworthy, in our series of SCD (47%) victims there was macroscopic evidence of myxomatous mitral valve and nearly half hal alff (4 (47% 7%)) ha 7% had a previous in vivo diagnosis of MVP, with a cardiological check-up ruling out channelopathies. Moreover, ECG, revision More reov re over ov er,, the er the EC CG, G which was available for revi visi vi sioon in 28%, did not sshow si how any evidence of ho long/short MVP ong g/short QT or Brugada Bru uga gada da syndromes. syn yndr drom dr omes om e . Of Of the the rremaining emaain nin ng 31 M VP ccases, ases as es,, 225 es 5 ((80%) 80%) 80 %) hhad a ffirstad irst st-st degree de egr gree e family famil illy members meemberrs referred mem refeerred ed for or cardiological caarddiolog ogiical screening, og sccreeening ng, without ng wi ho witho hout utt any anny evidence evi vide vi deence of channelopathies, MVP cases (16%). chan ch anne an nelo ne lopa lo path pa thie th iess, bbut ie utt M VP iin n 4 ca case sess (1 se (16% 6%)). 6% Although we are strong supporters of the relevance of molecular autopsy in the study of SCD21,22, we follow the indication by the HRS/EHRA/APHRS expert consensus statement52. According to these guidelines, an arrhythmia syndrome-focused postmortem genetic testing can be useful for all sudden unexplained death syndrome victims as Class IIa indication; furthermore, evaluation of first-degree blood relatives with resting ECG with high right ventricular leads, exercise stress testing, and echocardiography is recommended as Class I. Conclusions This study suggests that MVP is a significant cause of SCD in young adults and is the leading 15 DOI: 10.1161/CIRCULATIONAHA.115.016291 one in women. Arrhythmic MVP patients are mostly female with ventricular arrhythmias of LV origin and frequent repolarization abnormalities on inferior leads. The hallmark of arrhythmic MVP is fibrosis of PMs and infero-basal LV free wall, which well correlates with arrhythmia morphology, pointing to a myocardial stretch by the prolapsing leaflets and elongated chordae. CE-CMR allows the identification of this arrhythmic substrate and is a promising non-invasive tool for risk stratification and SCD prevention. Funding Sources: This work was supported by the Registry of Cardio- Cerebro-Vascular Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Pathology, Veneto Region, Venice, Italy; Target Project “Sudden cardiac death in women”, Regional Health System, Venice, Italy. Conflict of Interest Disclosures: None. References: Re efe ferrences re es es:: 11.. D Delling elling FN, V Vasan assan R RS. S. E Epidemiology pidemi pid mioolog ol gy andd ppathophysiology athopphys ysiiolo ys ogy y off m mitral itrall vvalve alvve prola prolapse: laps la pse: ne ps new ew insights molecular basis. Circulation. nsiigh g ts into disease d seeasse progression, di progresssio ion, genetics, io geene neticcs, aand ndd mo oleecu ula larr ba bas sis. Ci irculaatiionn. 22014;129:2158014;1229:21 211582170. 21 170 70. 22. Freed LA, LA Levy D, D Levine RA, RA Larson MG, MG Evans JC, JC Fuller DL, DL Lehman B, B Benjamin EJ. EJ Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med. 1999;341:1–7. 3. Freed LA, Benjamin EJ, Levy D, Larson MG, Evans JC, Fuller DL, Lehman B, Levine RA. Mitral valve prolapse in the general population: the benign nature of echocardiographic features in the Framingham Heart Study. J Am Coll Cardiol. 2002;40:1298-1304. 4. Nishimura RA, McGoon MD, Shub C, Miller FA Jr, Ilstrup DM, Tajik AJ. Echocardiographically documented mitral-valve prolapse. Long-term follow-up of 237 patients. N Engl J Med. 1985;313:1305-1309. 5. Düren DR, Becker AE, Dunning AJ. Long-term follow-up of idiopathic mitral valve prolapse in 300 patients: a prospective study. J Am Coll Cardiol. 1988;11:42-47. 6. Marks AR, Choong CY, Sanfilippo AJ, Ferré M, Weyman AE. Identification of high-risk and low-risk subgroups of patients with mitral-valve prolapse. N Engl J Med. 1989;320:1031-1036. 7. Avierinos JF, Gersh BJ, Melton LJ III, Bailey KR, Shub C, Nishimura RA, Tajik AJ, 16 DOI: 10.1161/CIRCULATIONAHA.115.016291 Enriquez-Sarano M. Natural history of asymptomatic mitral valve prolapse in the community. Circulation. 2002;106:1355-1361. 8. Davies MJ, Moore BP, Braimbridge MV. The floppy mitral valve. Study of incidence, pathology, and complications in surgical, necropsy, and forensic material. Br Heart J. 1978;40:468-481. 9. Kligfield P, Levy D, Devereux RB, Savage DD. Arrhythmias and sudden death in mitral valve prolapse. Am Heart J. 1987;113:1298-1307. 10. Vohra J, Sathe S, Warren R, Tatoulis J, Hunt D. Malignant ventricular arrhythmias in patients with mitral valve prolapse and mild mitral regurgitation. Pacing Clin Electrophysiol. 1993;16:387-393. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 11. Jeresaty RM. The syndrome associated with mid-systolic click and-or late systolic murmur. Analysis of 32 cases. Chest. 1971;59:643-647. 12. Shappell SD, Marshall CE, Brown RE, Bruce TA. Sudden death and the familiall occurrence 1973;48:1128-1134. of mid-systolic click, late systolic murmur syndrome. Circulation. 1973;48:1128-11 1134 34.. 34 13. Bharati S, Granston AS, Liebson PR, Loeb HS, Rosen KM, Lev M. The conduction system in n mitral valve prolapse syndrome with sudden death. Am Heart J. 1981;101:667-670. 14. Chesler E,, King RA, Edwards JE. The myxoma myxomatous valve 14 4. C hesler E matous mitral va ma alv lve and sudden death. Circulation. C irc rculation. 1983;67:632-639. rc 198 983; 98 3;677:6 :632 32-6 32 -639 -6 39 9. 15. WA, Bosman CK, Chesler Barlow JB, Edwards JE. Sudden 15 5. Pocock Po W A,, B osma man CK ma K, Ch C esle es l r E, E, Ba arllow JB B, Ed Edwa ard rds JE E. Su udd den ddeath eath ea t inn pprimary rim mary prolapse. 1984;107:378-382. mitral al vvalve alvve al ve pro ola laps pse. se Am m Heart Hea art J. J. 1984; 4;10 4; 107: 10 7:37 7: 37837 8 382. 38 82 16. Dollar AL, Roberts WC. Morphologic comparison of patients with mitral valve prolapse who died suddenly with patients who died from severe valvular dysfunction or other conditions. J Am Coll Cardiol. 1991;17:921-931. 17. Farb A, Tang AL, Atkinson JB, McCarthy WF, Virmani R. Comparison of cardiac findings in patients with mitral valve prolapse who die suddenly to those who have congestive heart failure from mitral regurgitation and to those with fatal noncardiac conditions. Am J Cardiol. 1992 ;70:234-239. 18. Morales AR, Romanelli R, Boucek RJ, Tate LG, Alvarez RT, Davis JT. Myxoid heart disease: an assessment of extravalvular cardiac pathology in severe mitral valve prolapse. Hum Pathol. 1992;23:129-137. 19. Burke AP, Farb A, Tang A, Smialek J, Virmani R. Fibromuscular dysplasia of small coronary arteries and fibrosis in the basilar ventricular septum in mitral valve prolapse. Am Heart J.1997;134:282-291. 17 DOI: 10.1161/CIRCULATIONAHA.115.016291 20. Corrado D, Basso C, Nava A, Rossi L, Thiene G. Sudden death in young people with apparently isolated mitral valve prolapse. G Ital Cardiol. 1997;27:1097-1105. 21. Basso C, Burke M, Fornes P, Gallagher PJ, de Gouveia RH, Sheppard M, Thiene G, van der Wal A; Guidelines for autopsy investigation of sudden cardiac death. Association for European Cardiovascular Pathology.Virchows Arch. 2008;452:11-18. 22. Basso C, Calabrese F, Corrado D, Thiene G. Postmortem diagnosis in sudden cardiac death victims: macroscopic, microscopic and molecular findings. Cardiovasc Res. 2001;50:290-300. 23. Levine RA, Stathogiannis E, Newell JB, Harrigan P, Weyman AE. Reconsideration of echocardiographic standards for mitral valve prolapse: lack of association between leaflet displacement isolated to the apical four chamber view and independent echocardiographic evidence of abnormality. J Am Coll Cardiol. 1988;11:1010-1019. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 24. Han Y, Peters DC, Salton CJ, Bzymek D, Nezafat R, Goddu B, Kissinger KV, Zimetbaum PJ, Manning WJ, Yeon SB. Cardiovascular magnetic resonance characterization of mitral valve prolapse. JACC Cardiovasc Imaging. 2008;1:294 2008;1:294-303. 303. 25. Perazzolo Marra M, De Lazzari M, Zorzi A, Migliore F, Zilio F, Calore C, Vettor Vet etto et torr G, Tona to Ton onaa F, Tarantini G, Cacciavillani L, Corbetti F, Giorgi B, Miotto D, Thiene G, Basso C, Iliceto S, Corrado D. Impact of the presence and amount of myocardial fibrosis by cardiac magnetic resonance esoona nanc ncee on arrhythmic nc arrrhy hythmic outcome and sudden cardiac caard rdia i c death in non ischemic ische hemi he m c dilated cardiomyopathy. caard rdiiomyopat io athy. Heart Rhythm. at Rhyt y hm. 2014;11:856-863. 2014;11:856-863 3. 26. Kelly KL, Titus Sudden with apparently normal heart. 2 . Chugh SS, K 26 ellly KL K L, Ti itu tus JL. Sudd dden ccardiac arrdiaac ddeath eath th w ithh ap ppaarenttly y no orm mal he hea ar art. Circulation. Circ Ci rcul rc u ation.. 22000;102:649-654. 00 00; 0;1022:6649-66544. 4. 27. 27 Topaz Topa To paz O, pa O, Edwards Edw dwar ards ar ds JE. JE. Pathologic Pat atho holo ho logi lo gicc features gi feat fe atur at ures res of of sudden sudde ud dde denn death deat de athh in children, at chi hild ldre ld renn, adolescents, re ado dole lesc le scen sc ents en ts, and ts and young adults. Chest. 1985;87:476-482. 28. Campbell RW, Godman MG, Fiddler GI, Marquis RM, Julian DG. Ventricular arrhythmias in syndrome of balloon deformity of mitral valve. Definition of possible high risk group. Br Heart J. 1976;38:1053-1057. 29. De Maria AN, Amsterdam EA, Vismara LA, Neumann A, Mason DT. Arrhythmias in the mitral valve prolapse syndrome. Ann Intern Med. 1976;84:656-660. 30. Winkle RA, Lopes MG, Popp RL, Hancock EW. Life-threatening arrhythmias in the mitral valve prolapse syndrome. Am J Med. 1976;60:961-967. 31. Levy S. Arrhythmias in the mitral valve prolapse syndrome: clinical significance and management. Pacing Clin Electrophysiol. 1992;15:1080-1088. 32. Morady F, Shen E, Bhandari A, Schwartz A, Scheinman MM. Programmed ventricular stimulation in mitral valve prolapse: analysis of 36 patients. Am J Cardiol. 1984;53:135-138. 18 DOI: 10.1161/CIRCULATIONAHA.115.016291 33. Sanfilippo AJ, Abdollah H, Burggraf GW. Quantitation and significance of systolic mitral leaflet displacement in mitral valve prolapse Am J Cardiol. 1989;64:1349-1355. 34. Kulan K, Komsuo÷lu B, Tuncer C, Kulan C. Significance of QT dispersion on ventricular arrhythmias in mitral valve prolapse. Int J Cardiol. 1996;54:251-257. 35. Devereux RB, Kramer-Fox R, Shear MK, Kligfield P, Pini R, Savage DD. Diagnosis and classification of severity of mitral valve prolapse: methodologic, biologic, and prognostic considerations. Am Heart J. 1987;113:1265-1280. 36. Lichstein E. Site of origin of ventricular premature beats in patients with mitral valve prolapse. Am Heart J. 1980;100:450-457. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 37. Sriram CS, Syed FF, Ferguson ME, Johnson JN, Enriquez-Sarano M, Cetta F, Cannon BC, Asirvatham SJ, Ackerman MJ. Malignant bileaflet mitral valve prolapse syndrome in patients with otherwise idiopathic out-of-hospital cardiac arrest. J Am Coll Cardiol. 2013;62:222-230. Kramer-Fox Natural 38. Zuppiroli A, Rinaldi M, Kramer Fox R, Favilli S, Roman MJ, Devereux RB. Nat tur u al history of mitral valve prolapse. Am J Cardiol. 1995;75:1028-1032. 39. Cobbs BW Jr, King SB 3rd. Ventricular buckling: a ffactor in the abnormal ventriculogram and peculiar hemodynamics associated with mitral valve prolapse. Am Heart J. 1977;93:741758.. 440. 0. Salazar AE, AE E, Edwards Edwa Ed ward wa rdss JE. rd JE. Friction Fric Fr icti ic tioon lesions ti lessionns of of ventricular veentricu cula cu larr endocardium. la end ndoc o ar ardi dium di um. Relation um Reela lati tion ti on to to chordae chorrda ch daee tendineae mitral valve. Arch Pathol. enddineae of mitra r l va ra alv lve. Ar rch h Pathol l. 11970;90:364-376. 9700;990:364 4-376 76.. 76 Barlow Bosman leaflet mitral valve. 41. Ba Barl rlow rl ow JB, B ossma mann CK. C . Aneurysmal CK Aneu An eurysm eu mal protrusion pro rotr trus tr ussion ion off tthe he pposterior osteri rioor ri or le eaf afle lett off tthe le he m itra rall valv ra va alv lvee. Heart An aauscultatory-electrocardiographic usscult uscu ltat lt ator at ory-el or elec el ectr ec troc tr ocar oc ardi ar diog di ogra og raph ra phic ph ic ssyndrome. ynndr yndr drom omee. Am om Am H eart rtt JJ.. 11966;71:166-178. 966; 96 6;71 6; 71:1 71 :166 :1 66-178 178 78. 42. Boudoulas H, Kolibash AJ, Baker P, King BD, Wooley CF. Mitral valve prolapse and the mitral valve prolapse syndrome: a diagnostic classification and pathogenesis of symptoms. Am Heart J. 1989;118:796-818. 43. Mason JW, Koch FH, Billingham ME, Winkle RA. Cardiac biopsy evidence for a cardiomyopathy associated with symptomatic mitral valve prolapse. Am J Cardiol. 1978;42:557562. 44. Jeresaty RM. Sudden death in the mitral valve prolapse-click syndrome. Am J Cardiol. 1976;37:317-318. 45. Wilde AA, Düren DR, Hauer RN, deBakker JM, Bakker PF, Becker AE, Janse MJ. Mitral valve prolapse and ventricular arrhythmias: observations in a patient with a 20-year history. J Cardiovasc Electrophysiol. 1997;8:307-316. 46. John BT, Tamarappoo BK, Titus JL, Edwards WD, Shen WK, Chugh SS. Global remodeling 19 DOI: 10.1161/CIRCULATIONAHA.115.016291 of the ventricular interstitium in idiopathic myocardial fibrosis and sudden cardiac death. Heart Rhythm. 2004;1:141-149. 47. Hancock EW, Cohn K. The syndrome associated with midsystolic click and late systolic murmur. Am J Med. 1966;41:183-196. 48. Scampardonis G, Yang SS, Maranhão V, Goldberg H, Gooch AS. Left ventricular abnormalities in prolapsed mitral leaflet syndrome. Review of eighty-seven cases. Circulation. 1973;48:287-297. 49. Rizzon P, Biasco G, Brindicci G, Mauro F. Familial syndrome of midsystolic click and late systolic murmur. Br Heart J. 1973;35:245-259. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 50. Gulotta SJ, Gulco L, Padmanabhan V, Miller S. The syndrome of systolic click, murmur, and mitral valve prolapse--a cardiomyopathy? Circulation. 1974;49:717-728. 51. Crawford MH, O'Rourke RA. Mitral valve prolapse: a cardiomyopathic state? Prog 1984;27:133-139. Cardiovasc Dis. 1984;27:133 139. 52. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, J, Chiang Chia Ch iang ia ng CE, CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. Executive summary: HRS/EHRA/APHRS expert consensus statement on the diagnosis management inherited arrhythmia diag gno nosi siss an si andd ma anagement na of patients with inher erit er ited it e primary arrhythmi miaa syndromes. Heart mi Rhythm. 2013;10:e85-108. Rh hyt ythhm. 201 0113;10:e85-108. 20 DOI: 10.1161/CIRCULATIONAHA.115.016291 Table 1. Clinical and pathologic features of 43 patients who died suddenly with MVP due to myxomatous degeneration. Variables Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Age, years median (range) Female, n (%) Athletes, n (%) Marfan stigmata, n (%) Pectus excavatum, n (%) Pregnancy, n (%) Circumstances of SCD, n (%) - on emotion/effort - at rest - during sleep 12 lead ECG available, n (%) nverted/biphasic T wave D2, D3, aVF n (%) Inverted/biphasic VAs, n (%) VAs morphology, n (%) - RBBB - LBBB Be Beta-blocker eta ta-b -blo -b lock lo cker ck er the therapy, era rappy, n (%) G Gross rooss featur features res H eart weight (g) ), m eaan ± SD D* Heart (g), mean ±SD* LV w all thickness thicckn k esss (mm) m), me ean n±SD D wall (mm), mean±SD VS S thickness thi hick ckne ck nesss (mm), ne (mm mm), ) m ), eaan SD ean± D mean±SD Pate Pa Patent tent te nt foramen for oram amen am en oovale, vale va le, n (% le (%)* )* O Ovall ffossa aneurysm, n (%) Posterior MVP, n (%) Bileaflet MVP, n (%) Endocardial fibrous plaque, n (%) Histology features LV scar - papillary muscles, n (%) -infero-basal wall, n (%) Fibrous tissue /myocardium (% area) - papillary muscles, mean ± SD† - infero-basal wall, mean ± SD† Cardiomyocytes diameter (μm), mean± SD† SCD due to MVP 43 patients 32 (19-40) 26 (61) 4 (9) 2 (5) 2 (5) 2/26 (8) Control 15 patients 30 (18-40) 10 (67) 2 (13) 0 0 1/10 p 0.33 0.7 1.0 1.0 1.0 1.0 8 (19) 30 (70) 5 (12) 12 (28) 10 (83) 12 (28) 4 (27) 9 (60) 2 (13) 5 (33) 0 0 1.0 1.0 1.0 - 12 (100) 8 (67) 9 (21) 0 0 0 - 357± 35 7± ±53 3 357±53 12 .6± 6±1..3 12.6±1.3 13 .0±0 .0 0±0.88 13.0±0.8 25 ((58) 58)) 58 10 (23) 13 (30) 30 (70) 25 (58) 323 23± 23 ± 42 323±42 12. .5± ±3.6 12.5±3.6 112.57±0.7 2 577±0 2. ±0.7 .7 4 (2 (27) 7) 1 (6) 0 0 0 00.02 .02 2 0.9 00.08 .008 00.04 .04 04 00.25 25 - 43 (100) 38 (88) 0 0 - 30.5±10.7 33.1±7.6 19.2±6.0 6.3±1.6 6.4±1.4 12.8±0.4 <0.0001 <0.0001 <0.0001 Abbreviations: LV= Left ventricle; MVP= mitral valve prolapse; RBBB= right bundle branch block; SCD= sudden cardiac death; VAs= ventricular arrhythmias; VS= ventricular septum. 21 DOI: 10.1161/CIRCULATIONAHA.115.016291 Table 2. Clinical, ECG and CMR features of 44 patients with MVP. Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Variables Age, years median (range) Female, n (%) Symptoms, n (%) - aborted SCD - palpitations tations - syncope ope - chest pain - dyspnea nea Therapy, n (%) Beta-blockers -blockers Sotalol lol Otherr antiarrhythmic anti tiar arrh ar rhyt rh ythm yt hmic hm ic ICD G 12 lead ECG Inverted/biphasic pha h sic ic T wave, n (%) D , aV D3 aaVF F - D2, D3, - D1, aV aVL V QTc duration, n, msec se onit on itor it orin or ingg in ECG-Holterr m monitoring VPB,, n (%) - Bigeminal mina mi nall VP na VPB B - NSVT, T n (%) SVT, n (%) VF, n (%) CVAs morphology, n (%) - LBBB inferior axis - LBBB superior axis - RBBB inferior axis - RBBB superior axis CMR Morpho-functional Findings LV EDV, ml/m2 LV EF, % MVP with Complex VA 30 pts Complex VA >3 VPB run 10 pts Complex VA =3 VPB run 20 pts MVP without Complex VA 14 pts 41 (28-43) 22 (73) 37 (32-43) 9 (90) 44 (36-52) 13 (65) 51 (24-64) 7 (50) 3VPB vs without Complex VA 0.44 0.18 2 ((7)) 15 (50) 2 (7) 2 (7) 2 (7) 2 (20) ( ) 7 (70) 2 (20) 0 1 (10) 0 8 (40) 0 2 (10) 1 (5) 0 5 (36) 0 1 (7) 1 (7) 0.52 1.00 1.00 1.00 0.21 1.63 1.00 1.00 1.00 1.00 1.00 1.00 1.00 0.24 0.10 0.54 1.00 13 (43) 3 (10) 1 (3) 3 (10) 5 (50) 1 (10) 1 (10) 3 (100) 8 (40) 2 (10) 0 0 6 (43) 0 1 (7) 0 1.00 0.54 0.54 - 1.00 0.42 1.00 - 1.00 0.50 0.41 - 0.71 1.00 0.33 - 100 (33) (33 3 ) 9 (30) 0 2 (7) 7) 4423 23 3 (409(409-440) 9 440) 4 5 (50) 50 4 (40) 0) 2 (20) 4439 39 9 ((420-446) 420-44 446) 44 5 5 (25) 5 5 (25) 0 420 ((409-431) 4 940 9 431) ( 1)) (2 3 (21) ( 4) (1 2 (14) (7) 1 (7) 4412 12 2 (39 (394-432) 3 4-43 4 2)) 0.55 0.5 0.46 1.00 0.19 0.220 0. 0.20 0 19 0. 0.19 0 55 0. 0.55 00.15 0. 15 00 1.00 0.67 0.41 00.34 .34 0.23 0.43 0.10 0.18 30 (100) 11 ((37) 37)) 37 27 (90) 1 (3) 2 (7) 100 ((100) 100) 5 (5 (50) 0) 7 (70) 1 (10) 2 (20) 20 (1 ((100) 00) 6 ((30) 30)) 30 20 (100) 0 0 8 (57) 3 (2 (21) 1) 0 0 0 <0.01 00.49 .49 49 - 0.022 00.20 .20 20 - <0.01 00.70 .70 70 - 0.43 - 0 1 (3) 13 (43) 26 (87) 0 0 7 (70) 10 (100) 0 1 (5) 5 (25) 16 (80) 0 0 0 0 - - - 1.00 0.05 0.27 91 (89-103) 64 (60-65) 91 (91-94) 63 (59-65) 91 (89-108) 64 (59-65) 91 (83-91) 66 (64-69) 0.13 <0.01 0.24 <0.01 0.18 0.01 0.91 0.68 22 p Value > 3VBP =3VPB vs vs without without Complex VA Complex VA 0.40 0.59 0.08 0.49 >3VPB vs = 3 VPB 0.27 0.21 DOI: 10.1161/CIRCULATIONAHA.115.016291 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 LV mass, gr/m2 RV EDV, ml/m2 RV EF, % Posterior MVP, n (%) Bileaflet MVP, n (%) Lengths MV leaflets, mm - Anterior - Posterior Prolapse distance, mm - Anterior leaflet - Posterior leaflet contrast Findings CMR Post-contrast LV LGE - papillary lary muscles - infero-basal o-basal wall mount (%) LV LGE amount 62 (60-63) 77 (71-79) 64 (61-66) 9 (30) 21 (70) 62 (59-74) 77 (71-79) 65 (62-69) 5 (50) 5 (50) 62 (60-63) 77 (75-81) 64 (62-65) 4 (20) 16 (80) 63 (49-63) 77 (76-78) 64 (64-66) 9 (64) 5 (36) 20.7 (19.3-26.0) 20.1 (18.5-28.0) 22.1 (20.0-25.0) 20.0 (17.0-25.0) 16.0 (12.6-18.0) 14.0 (11.0-17.7) 16.3 (13.0-19.7) 11.4 (9.5-14.0) 0.48 0.35 0.43 0.05 0.05 0.55 0.51 0.93 0.68 0.68 0.57 0.39 0.27 0.01 0.01 0.75 0.91 0.31 0.12 0.12 0.32 0.02 0.51 0.26 0.34 0.01 0.65 0.27 5.1 (1.7-8.0) 7.8 (4.0-11.8) 3.3 (0-7.0) 4.5 (2.7-7.5) 5.6 (3.9-8.0) 10 (5.5-12.9) 1.3 (0-3.0) 2.1 (2.0-3.5) 0.01 <0.01 0.47 0.11 <0.01 <0.01 0.12 <0.01 28 (93) 25 (83) 22 (73) 1.2 (0.8-2.1) 10 (100) 10 (100) 7 (70) 1.1 (0.9-2.7) 18 (90) 15 (75) 15 (75) 1.4 (0.7-2.1) 2 (14) 2 (14) 1 (7) 0 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 <0.01 <0.0 <0 .01 01 <0.01 <0.0 <0 .011 .0 01 <0.01 <0 <0.01 0.54 0.14 1.00 0.96 Abbreviations: s: CMR=cardiac magnetic resonance; VA= ventricular arrhythmias; EDV = End-Diastolic Volume; EF= Ejection Fraction; ICD = Implantable cardioverter defibrillator; defibrillat LGE= late gadolinium corrected; um enhancement; LBBB= left bundle branch block; LV= Left Ventricle;MVP= mitral valve prolapse; NSVT = non-sustained ventricular tachycardia; QTc = QT corre RBBB= right bundle branch block; RV= right rig ight ventricle; SCD= sudden cardiac death; SVT= sustaine sustained ned ventricular tachycardia; VPB=ve ne VPB=ventricular ent n ricular premature beats; VF = ventricular fibrillation. Categorical variables ariab ble less ar aree pr pres presented esen es ente en tedd as num te number u be b r of patients (%). Continuous values are express expressed ed d aass me m median dian with 25% and 75%-iles. 23 DOI: 10.1161/CIRCULATIONAHA.115.016291 Figure Legends: Figure 1. SCD in a 36 years old woman with in vivo diagnosis of MVP. A,B) 12-lead basal ECG at the time of emergency department admission for palpitations. Single and coupled VPBs with RBBB morphology are present; note the negative T wave on the inferior leads. At 24 hour Holter-ECG (B) NSVT is also recorded; C) At gross examination, myxomatous degeneration of both leaflets of the mitral valve with elongated chordae is visible; D, E). At histology, severe Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 myxoid thickening of the posterior mitral valve leaflet and myocardial fibrosis of the LV inferobasal wall (D) and PM (E). Figure 2.Histology of three representative SCD cases with MVP. Myocardial scarring is visible underneath mitral at the hee level lev evel el ooff thee in iinfero-basal fero-basal LV free wall, unde dern de rneath the posterior m rn itra it r l valve leaflet (A,B,C) and and of the PMss plus plu us adjacent adja ad jace ace cent nt free freee wall wal alll (D,E,F). (D D,E E,F ,F)). Close-up Clo ose-u -u up of the t e scarring th s ar sc arri ring ri ng aareas reas as sshowing howi ho wing wi ng eendondond operimysial replacement-type fibrosis with cardiomyocytes peeri r mysial my aand n ppatchy nd attchyy re eplaccem mentt-ttyppe fib tbrossis w itth iinterspersed nterssper nt erse er seed ca ard r io iomy myoc my ocyt oc y ess ((G,H,I). G,H H,II). Figure 3. CMR post-contrast sequences findings in MVP patients with complex ventricular arrhythmias and aborted SCD. A,B) A30 years old woman with MVP and complex ventricular arrhythmias. LGE of the PM is visible on mid-short axis view (A). The 12 lead ECG (B) shows the presence of NSVT with RBBB morphology originating from the posterior PM (superior axis). C,D) A 33 years old woman with MVP and complex ventricular arrhythmias. LGE of the LV infero-basal region, underneath the posterior valve leaflet, with endocardial-midmural extension, is visible on 3-chamber long axis view (C). The 12 lead ECG demonstrates NSVT with RBBB morphology originating from the LV infero-basal wall near the mitral annulus 24 DOI: 10.1161/CIRCULATIONAHA.115.016291 (inferior axis) (D). E, F) A 38 years old man with MVP and aborted SCD. CMR, performed 6 months before cardiac arrest, shows LGE in the infero-basal region of the LV on long-axis view (E). ECG recording of polymorphic SVT degenerating into VF (F). Figure 4. A 34 year old woman with pre-syncopal episodes despite antiarrhythmic drug therapy. A) Basal ECG shows isolated VPBs with two RBBB morphologies, indicating a LV origin from the PMs and the infero-basal wall close to the mitral anulus. B,C) Electro-physiologic study Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 with programmed stimulation and induction of sustained VT with the same morphology of VPBs originating from the posterior mitral annulus, terminated by electrical cardioversion. D) On CECMR, LGE at the level of the LV infero-basal wall is visible. 25 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Figure 1 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Figure 2 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Figure 3 Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Figure 4 Arrhythmic Mitral Valve Prolapse and Sudden Cardiac Death Cristina Basso, Martina Perazzolo Marra, Stefania Rizzo, Manuel De Lazzari, Benedetta Giorgi, Alberto Cipriani, Anna Chiara Frigo, Ilaria Rigato, Federico Migliore, Kalliopi Pilichou, Emanuele Bertaglia, Luisa Cacciavillani, Barbara Bauce, Domenico Corrado, Gaetano Thiene and Sabino Iliceto Downloaded from http://circ.ahajournals.org/ by guest on October 1, 2016 Circulation. published online July 9, 2015; Circulation is published by the American Heart Association, 7272 Greenville Avenue, Dallas, TX 75231 Copyright © 2015 American Heart Association, Inc. All rights reserved. Print ISSN: 0009-7322. Online ISSN: 1524-4539 The online version of this article, along with updated information and services, is located on the World Wide Web at: http://circ.ahajournals.org/content/early/2015/07/09/CIRCULATIONAHA.115.016291 Permissions: Requests for permissions to reproduce figures, tables, or portions of articles originally published in Circulation can be obtained via RightsLink, a service of the Copyright Clearance Center, not the Editorial Office. Once the online version of the published article for which permission is being requested is located, click Request Permissions in the middle column of the Web page under Services. Further information about this process is available in the Permissions and Rights Question and Answer document. Reprints: Information about reprints can be found online at: http://www.lww.com/reprints Subscriptions: Information about subscribing to Circulation is online at: http://circ.ahajournals.org//subscriptions/