* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download drug metabolism
Plateau principle wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Polysubstance dependence wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Orphan drug wikipedia , lookup
Compounding wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Psychopharmacology wikipedia , lookup
Theralizumab wikipedia , lookup
Neuropharmacology wikipedia , lookup
Drug design wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Prescription costs wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmacognosy wikipedia , lookup
Pharmacokinetics wikipedia , lookup
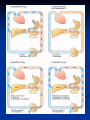
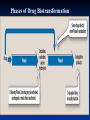
Drug Elimination Drug elimination consists of 2 processes Drug Metabolism or Biotransformation Drug Excretion METABOLISM Biotransformation, or drug metabolism, is the enzyme-catalyzed conversion of drugs to their metabolites. Most liver. drug biotransformation takes place in the Clinical sketch A man with moderately severe alcoholic liver disease needs flecainide for a cardiac arrhythmia. The clinical team consults Appendix of the BNF and is advised to give reduced doses. Comments: flecainide accumulates in patients with chronic liver disease. WHY IS DRUG BIOTRANSFORMATION NECESSARY? The majority of drugs are lipidsoluble to some extent. Most lipid-soluble drugs filtered through the glomerulus are largely reabsorbed into the systemic circulation during passage though the renal tubules. lipid-soluble drug water-soluble drug WHY IS DRUG BIOTRANSFORMATION NECESSARY? The end result of metabolism is that the original drug molecule is altered in ways that make the drug more polar, hydrophilic, and water soluble Without metabolism, 99.9% of all drugs filtered at the glomerulus would be reabsorbed into systemic circulation. Role of Drug Biotransformation The fundamental role of drug-metabolizing enzymes is to inactivate and detoxify drugs and other foreign compounds (xenobiotics). Drug metabolites are usually more water soluble than is the parent molecule. Role of Drug Biotransformation No particular relationship exists between biotransformation and pharmacologic activity. Most conjugated drug metabolites are inactive, but a few exceptions exist. Formation of Active Metabolites Some agents, known as prodrugs, are administered as inactive compounds and then biotransformed to active metabolites. Paracetamol poisoning Toxic metabolites of paracetamol are detoxified by phase II conjugation joining with glutathione. In an overdose situation, toxic metabolites accumulate causing toxicity and can result in hepatitis. Phases of Drug Biotransformation General Metabolic Pathways Phase I reactions – Functionalization Oxidation Reduction Hydrolytic reactions Purpose Introduction of polar functional groups in a molecules Hydroxyl groups; Carboxylate groups; Amino Groups; Thiol Groups Increase a molecules polarity Does provide a site for phase II metabolism Phase I reactions Oxidative Reactions Oxidative reactions are catalyzed by enzymes isolated in the microsomal fraction of liver homogenates (the fraction derived from the endoplasmic reticulum) and by cytoplasmic enzymes. Cytochrome P450s are the enzymes that catalyze phase I reactions. Cytochrome P450 Monooxygenase System A family of enzymes that catalyze the biotransformation of drugs Modest specificity for substrates and catalyze the metabolism of widely differing chemical structures Characterized by an intense absorption band at 450 nm in the presence of carbon monoxide. Phase I reactions family ≥40% subfamily≥55% ROLE OF CYP ENZYMES IN HEPATIC DRUG METABOLISM RELATIVE HEPATIC CONTENT OF CYP ENZYMES CYP2D6 2% % DRUGS METABOLIZED BY CYP ENZYMES CYP2E1 7% CYP 2C19 11% CYP 2C9 14% CYP2D6 23% CYP 2C 17% OTHER 36% CYP 1A2 12% CYP 3A4-5 26% CYP 1A2 14% CYP 3A4-5 33% CYP2E1 5% Cytochrome P450 Monooxygenase System Genetic polymorphisms exist for these genes Genetic variations in these metabolism genes can have important consequences for patients. “Poor metabolizers” have a greater risk than the general population of experiencing adverse drug reactions. Hydrolysis of Esters and Amides Catalyzed by widely distributed hydrolytic enzymes Cholinesterase and other plasma esterases Few CYP enzymes that carry out hydrolytic reactions Phase I reactions Phase I reactions Reductive Reactions Less common than are oxidative and hydrolytic reactions. Nitroglycerin, a vasodilator, undergoes reductive hydrolysis catalyzed by glutathione- organic nitrate reductase. General Metabolic Pathways Phase II reactions – Conjugation Purpose Introduce highly polar conjugates Glucuronic acid; Sulfate; Glycine or other Amino Acids; Glutathione Conjugation enzymes are present in the liver and other tissues Conjugates are readily excreted in the urine Usually inactive - notable exception is morphine 6-glucuronide Enzyme Induction and Inhibition Many drugs alter drug metabolism by inhibiting or inducing CYP enzymes. Drug interactions can occur when these drugs are administered concurrently with other drugs that are metabolized by CYP. Enzyme Induction Enzyme induction is the process by which exposure to certain substrates results in accelerated biotransformation with a corresponding reduction in parent drug. Enzyme inducer stimulates the synthesis of the enzyme and the metabolic capacity is increased Consequences of Induction Increased rate of metabolism Decrease in drug plasma concentration Enhanced oral first pass metabolism Reduced bioavailability If metabolite is active or reactive, increased drug effects or toxicity Clinical sketch A young woman on the contraceptive pills is found to have tuberculosis. She is started on treatment and suffers contraceptive failure soon after. Comments: it would be unacceptable practice to fail to warn the woman that rifampicin (which induces the liver drugmetabolising enzyme CYP450) is very likely to cause failure of contraception. Therapeutic Implications of Induction Most drugs can exhibit decreased efficacy due to rapid metabolism but drugs with active metabolites can display increased drug effect and/or toxicity due to enzyme induction Dosing rates may need to be increased to maintain effective plasma concentrations Induction Auto-inducer Some currently used drugs are well known to induce their own metabolism or the metabolism of other drugs. The longer the drug is given, the more rapidly it is metabolized. Enzyme Inhibition Enzyme inhibitor inhibits the synthesis of the enzyme and the metabolic capacity is decreased Cimetidine: CYP2C9 P450 enzyme inhibitor Grapefruit + Statins Consequences of Inhibition Increase in the plasma concentration of parent drug Exaggerated and prolonged pharmacological effects Increased likelihood of drug-induced toxicity Enzyme induction will decrease the duration and intensity of the drug action. The opposite is also true. In cases where an enzyme is responsible for metabolizing a pro-drug into a drug, enzyme induction can speed up this conversion and increase drug levels, potentially causing toxicity.