* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download File
Survey
Document related concepts
Transcript
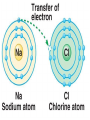
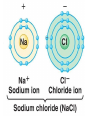
2.1 – Matter all objects are made of matter ~ something with mass and volume (remember the density lab?) matter can usually be found on Earth as a solid, liquid, or a gas atom: building block of all matter atoms are made up of smaller parts: proton: particle that has a positive (+) charge ~ large in size neutron: particle that has no charge (neutral) ~ large in size (like a proton) electron: particle that has a negative (-) charge ~ very small ~ about 1/1800th the size of a proton or neutron atoms have no overall charge (neutral) because there is an equal number of protons (+) and electrons (-) nucleus: center of atom ~ contains protons and neutrons electron cloud: area of space that surrounds the nucleus ~ contains the electrons electron cloud nucleus Happy, happy Carbon atom! But, are all parts really happy? element: substance that can’t be broken down into a simpler form elements are organized by their properties on the periodic table (p.36) 95 naturally occurring elements with only 8 making up most of Earth’s crust element symbols are usually 1 or 2 letters ~ hydrogen = H, carbon = C, oxygen = O each element has a different number of protons ~ hydrogen (H) = 1, carbon (C) = 6, oxygen (O) = 8 number of protons is called the atomic number of the element in nature, there can be several versions of the same element same number of protons BUT have a different number of neutrons ~ called isotopes some isotopes are unstable and give off neutrons which makes them radioactive Hydrogen – 1 P, 0 N Hydrogen 2 (Deuterium) – 1 P, 1 N Hydrogen 3 (Tritium) – 1 P, 2 N radioactive isotope isotopes of the same element are shown by writing the symbol and the mass number ~ H-1, H-2, H-3 mass number: total number of protons and neutrons in an atom ~ remember those are the two particles with any measurable mass ~ electrons are too small! Hydrogen – 1 P, 0 N H-1 Hydrogen 2 (Deuterium) – 1 P, 1 N H-2 Hydrogen 3 (Tritium) – 1 P, 2 N H-3 The NEW Periodic Table https://www.youtube.com/watch?v=zUDDiWt FtEM Read the lyrics to follow along! compound: substance made from atoms of 2 or more different elements atoms are held together by a force called a chemical bond arrangement of electrons determines how atoms bond together electrons in the electron cloud are grouped in different energy levels each level can hold a limited number of electrons outer level can hold 8 electrons (except for H and He) ~ is stable with outer level full if the outer level ISN’T full, the atom will bond with other atoms to get a total of 8 in the outer shell sometimes atoms will lose or gain electrons in order to get a total of 8 electrons in their outer shell atoms will then have a charge due to an unequal number of electrons (-) and protons (+) ~ those atoms are called ions atoms that lose electrons are positive ions (more P than e) atoms that gain electrons are negative ions (more e than P) opposite charges attract so an ionic bond forms between opposite ions, creating crystalline ionic compounds covalent bond: when atoms share electrons to form a covalent compound since the number of protons (+) is equal to the number of electrons (-), the charge of the molecule is neutral (no charge) metallic bond: when atoms share electrons between many metal ions metal ions are positive so are attracted to the negative electrons because many ions are sharing many electrons, metals have unique properties: easily shaped (malleable) drawn into thin wires conduct heat and electricity