* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Applications of Solid-Liquid Phase Change - Thermal
Glass transition wikipedia , lookup
Energy applications of nanotechnology wikipedia , lookup
Shape-memory alloy wikipedia , lookup
State of matter wikipedia , lookup
Superconductivity wikipedia , lookup
Thermodynamic temperature wikipedia , lookup
Condensed matter physics wikipedia , lookup
Heat transfer physics wikipedia , lookup
Work (thermodynamics) wikipedia , lookup
Sol–gel process wikipedia , lookup
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9 Applications of Solid-Liquid Phase Change
6.9.1 Latent Heat Thermal Energy Storage
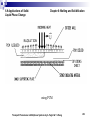
The theoretical model employed in this study is shown in
Fig. 6.31. At the very beginning of the process (t =0), the
tube, which has a radius of R0, is surrounded by a liquid
phase change material with uniform temperature Tf>Tm.
The temperature of the working fluid inside the tube is Ti
and the convective heat transfer coefficient between the
working fluid and the internal tube wall is hi.
Both hi and Ti are kept constant throughout the process.
The thickness of the tube is assumed to be very thin, so
the thermal resistance of the tube wall can be neglected.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
1
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.31 Solidification around a horizontal tube
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
2
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The phase change material can be treated
as if it were directly in contact with the
coolant inside the tube.
Liquid adjacent to the cooled surface will
be solidified, and the temperature
difference between the solid-liquid
interface and the otherwise quiescent
liquid will drive natural convection in the
liquid region.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
3
6.9 Applications of SolidLiquid Phase Change
1.
2.
3.
4.
5.
Chapter 6: Melting and Solidification
The liquid is Newtonian and Boussinesq as well as
incompressible. The Prandtl number of the liquid
phase change material is larger.
The solid is homogeneous and isotropic.
The liquid motion induced by volumetric variation
during solidification is neglected, i.e., the density of the
solid is equal to the density of the liquid. In addition,
the phase change material’s properties are constant in
the liquid and solid regions.
The solid-liquid interface is assumed to be a smooth
cylinder concentric with the cooled tube.
The effect of natural convection is restricted within the
boundary layer and the bulk liquid has a uniform
temperature, Tf.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
4
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The boundary layer equations of the problem
can be written as follows:
∂u ∂v
+
= 0
∂x ∂y
(6.358)
∂ 2u
ν
+ g β (T − Tm )sin ϕ = 0
2
∂y
(6.359)
∂ T ∂ (uT ) ∂ (vT )
+
+
=α
∂t
∂x
∂y
(6.360)
f
∂ 2T
∂ y2
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
5
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Since the Prandtl number of the liquid is much
larger than unity, the inertia terms in the
momentum equation have been neglected.
These equations were solved by an integral
method. Assuming a polynomial profile, the
temperature and velocity profiles inside the
boundary layer of thickness are expressed as
y
T = T f − (T f − Tm ) 1 −
δ
y
y
u = U 1−
δ
δ
2
(6.361)
2
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
(6.362)
6
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
g β sin ϕ
U=
(T f − Tm )δ
3ν
1 ∂ δ dR 1
g β sin ϕ ∂ 3δ 2α
−
+
−
(T f − Tm )
+
3
3 ∂ t dt 90
ν R ∂ϕ
δ
(6.363)
2
f
= 0 (6.364)
Heat transfer in the solidified layer is dominated
by conduction. The governing equation of the
solid layer and corresponding boundary
conditions are as follows:
1 ∂ ∂T 1 ∂T
R0 < r < R t > 0 (6.365)
r
=
r ∂r ∂r α s ∂t
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
7
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
∂T
ks
= hi (T − Ti ) r = R0
∂r
Ts (r , t ) = Tm
∂ Ts
ks
∂r
∂T
− kf
∂y
r= R
y= 0
r= R t> 0
dR
= ρ hsl
dt
t> 0
(6.366)
(6.367)
r = R t > 0 (6.368)
Equations (6.364) – (6.368) can be nondimensionalized
and solved using integral approximate method.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
8
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
When Bi → ∞ , it corresponds to boundary conditions of
the first kind, i.e., the tube wall temperature is and is
kept steady throughout the process.
Wang et al. (1991) experimentally investigated the
solidification process around a horizontal cooled tube.
A comparison of the predicted solidification rate,
V / V0 = ( R / R0 ) 2 and the experimental results is shown in
Fig. 6.32.
When Ra = 0, i.e., no superheat exists in the liquid
region or the solidification process is dominated by
conduction, the predicted value is 18% lower than the
experimental data.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
9
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.32 Comparison of predicted solidification rate with
experiments.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
10
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
During the conduction dominated freezing process, the
front of the freezing layer is a dendritic layer, as
indicated by Wang et al. (1991).
Therefore, it is believed that the solid-liquid interface is
extended by the dentric layer.
As the Rayleigh number increases, natural convection
occurs in the liquid region, and the solid-liquid interface
becomes smooth because of the natural motion of the
liquid.
When Ra = 1.8x105, the predicted value is only 8% lower
than the experimental data, so the agreement is
satisfactory. The effect of Biot number on the wall
temperature and solidification rate was also studied by
Zhang et al. (1997).
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
11
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9.2 Heat Pipe Startup from Frozen State
When a high-temperature heat pipe starts from room
temperature, the working fluid within the wick structure is
in the frozen state.
During startup from the frozen state, large thermal
gradients generate significant internal stresses within the
pipe wall.
These stresses may severely shorten the life of the heat
pipe.
As a result, information on the stresses is needed for
design purposes, and it is necessary to determine the
temperature distribution during the transient frozen
startup period.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
12
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.33
Evolution of heat pipe
startup process from
the frozen
state (not to scale;
Cao and Faghri,
1993a).
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
13
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The startup process of a liquid metal heat pipe from the
frozen state may be divided into several periods for
convenience of analysis:
1.
2.
The heat pipe wick is initially in the frozen state. The heat pipe
core can be considered to be completely evacuated before
startup. The heat pipe in its initial state is illustrated in Fig. 6.33
(period 1).
Power is applied to the evaporator section of the heat pipe.
Heat conduction through the wall and melting of the working
fluid in the porous wick take place. However, the liquid-solid
melting interface has not yet reached the wickvapor boundary,
and the heat pipe core is still evacuated because no
evaporation has taken place in the evaporator section (Fig.
6.33, period 2).
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
14
6.9 Applications of SolidLiquid Phase Change
1.
2.
3.
Chapter 6: Melting and Solidification
In the evaporator section, the working fluid in the wick is
completely melted, and evaporation takes place at the liquid
vapor interface. The vapor pressure is so low that the vapor
flow is in a rarefied or free molecular condition. Also, some
working fluid in the wick is still in the solid state in the adiabatic
and condenser sections of the heat pipe (Fig. 6.33, period 3).
As evaporation continues, the amount of vapor accumulated in
the core is large enough in the evaporator that a continuum
flow is established there. Near the condenser end of the heat
pipe, however, the vapor flow is still in the rarefied or free
molecular regime. This period may be called the intermediate
period (Fig. 6.33, period 4).
The working fluid in the wick is completely melted and
continuum flow is established over the entire heat pipe length.
The heat pipe continues to operate and gradually reaches
steady state (Fig. 6.33, period 5).
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
15
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
For the porous wick structure, the change of phase of
the frozen working fluid must be considered.
The wick structure is assumed to be isotropic and
homogeneous.
The temperature transforming model (Cao and Faghri,
1990a) is employed to study melting of working fluid
during the heat pipe startup.
The energy equation in the wick structure (a porous
medium) is
∂
∂
∂T 1 ∂
∂T
( ρ eff cT ) =
keff
+
keff r
∂t
∂z
∂z r ∂r
∂r
∂
− ( ρ eff b) (6.369)
∂t
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
16
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The coefficients c, b, and keff in eq. (6.369) are
cse
c(T ) = cm + hsl /(2∆ T )
c
le
T < Tm − ∆ T
Tm − ∆ T < T < Tm + ∆ T
(6.370)
T > Tm + ∆ T
T < Tm − ∆ T
cse (∆ T − Tm )
b(T ) = cme ∆ T + hsl / 2 − ( cme + hsl /(2∆ T ) ) Tm Tm − ∆ T < T < Tm + ∆ T
c ∆T + h − c T
T > Tm + ∆ T
sl
le m
se
(6.371)
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
17
6.9 Applications of SolidLiquid Phase Change
keff
Chapter 6: Melting and Solidification
kse
= kse + (kle − kse )(T − Tm + ∆ T ) /(2∆ T )
k
le
T < Tm − ∆ T
Tm − ∆ T < T < Tm + ∆ T
T > Tm + ∆ T
where ρ eff = ϕ ρ l + (1 − ϕ ) ρ ws , cse = ϕ cs + (1 − ϕ )cws ,
cle = ϕ cl + (1 − ϕ )cws and cme = 0.5(cse + cle ) . The
values of k se and kle depend on the particular heat
pipe wick structure.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
18
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
(6.372)
Figure 6.34 Outer wall temperature compared with experimental
data for case 11a-11f
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
19
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9.3 Thermal Protection from Intense
Localized Heating Using PCM
In some applications, a surface is hit by a moving high
intensity heat source, as shown in Fig. 6.35.
The major concern here is how to protect the surface
from being burned out by the moving heat flux.
This is indeed a concern in laser thermal threats and reentry situations.
Ablation and heat pipe technologies are usually sources
of protection for surfaces in danger of being burned out
by a high heat flux.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
20
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.35 Schematic of localized heating
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
21
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Phase-change materials (PCMs) have a large melting heat, so then
offer an efficient means of absorbing the heat energy while the
materials are subjected to heat input, and releasing it afterward at a
relatively constant temperature.
Another alternative, as shown in Fig. 6.36, advantageously
incorporates the merits of the above technologies as protection for
surfaces attacked by a high heat flux.
There, the incoming heat input moves along the surface with speed
U and the heat is conducted through the outside wall to the PCM.
The PCM beneath the surface melts and absorbs a large amount of
the incoming heat.
Because of the large melting latent heat of the PCM and the
constant melting temperature Tm, the peak wall temperature will be
maintained at a temperature moderately higher than Tm.
With a low or moderate Tm, the reduction of the peak wall
temperature is evident.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
22
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
using PCM
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
23
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The dividing sheet, the soft insulating material and the supporting
plate may be used to prevent the PCM from separating from the
surface wall during the melting process, and to prevent the incoming
heat from being conducted into the cabin.
An analysis in a fixed coordinate system is difficult for this problem.
It is convenient to study it in a moving coordinate system where the
origin is fixed at the heated spot.
For an imaginary observer riding along with the incoming heat
beam, the outside wall and PCM will travel by at the same speed, U.
The energy equation in the Cartesian coordinate system for this
problem is (Cao and Faghri, 1990b)
∂h
∂h ∂ ∂T
ρ
− ρU
=
k
∂t
∂x ∂x ∂x
∂ ∂T
k
+
∂y ∂y
∂ ∂T
+
k
∂
z
∂
z
(6.373)
where the second term on the left-hand side is a convection term,
while the terms on the right-hand side are diffusive terms.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
24
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
25
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
∂ ( ρ h) 1 ∂ (rvr ρ h) 1 ∂ (vθ ρ h)
+
+
∂t
r
∂r
r ∂θ
1 ∂ ∂ (Γ h) 1 ∂ 1 ∂ (Γ h ) ∂ ∂ (Γ h )
=
r
+
+
r ∂x
∂ r r ∂ θ r ∂ θ ∂ z ∂ z
1 ∂ ∂ S 1 ∂ 1 ∂ S ∂ 2S
+
r +
+ 2
r ∂x ∂r r ∂θ r ∂θ ∂z
(6.374)
where vr = − U cosθ and vθ = U sin θ are the velocity components in
the cylindrical coordinate system, and Γ and S for the PCM can be
obtained from eqs. (6.224) and (6.225).
For the wall, no phase change occurs, and therefore h = cwT , Γ = k w / cw
and S=0. Equation (6.375) with corresponding boundary conditions
is solved using a finite volume method.
The resulting algebraic equations are solved with the Gauss-Siedel
method.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
26
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9.4 Microwave Thawing of Food and
Biological Materials
Food thawing processes are becoming more important
because the demand for frozen food products
continuously increases.
The interest in microwave thawing is stimulated by its
ability to avoid some of the disadvantages associated
with conventional thawing:
long processing times,
large space requirements,
microbial problems,
chemical deterioration,
drip loss,
high fresh water consumption.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
27
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
During microwave heating, the transient temperature
distribution within the material is determined by the internal
heat generation attributed to dissipation of electrical energy
from microwave radiation, and the heat transfer by conduction
and convection.
The moisture transfer within the material and evaporation at
the surface can also influence the temperature profile.
Modeling microwave thawing heat transfer is difficult due to
the effects of highly nonlinear phenomena, such as the rate of
energy dissipation and the energy distribution within the
material.
These phenomena are governed by the thermal, electrical,
and physical properties of the material, and vary with
temperature during the microwave thawing process.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
28
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Microwaves are electromagnetic waves with wavelengths
ranging from 1 cm to 1 m. The heat generation within the
microwave-irradiated frozen materials results from dipole
excitation and ion migration.
There may also be other mechanisms of interaction between
the material and the electromagnetic field which cause the
dissipation and heating effects of microwave energy.
The equations governing the absorption of microwave
radiation by a conducting material are the Maxwell equations
of electromagnetic waves.
In the time-varying case, the different field equations are
coupled, since a changing magnetic flux induces an electric
field and a time-varying electric flux induces a magnetic field.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
29
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
∂B
+ ∇ × E= 0
∂t
ε
(6.375)
∂E
+ σ EΗ
= ∇ ×
∂t
(6.376)
∇ ⋅ (ε Η ) = 0
(6.377)
∇ ⋅B= 0
(6.378)
B= µH
(6.379)
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
30
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
where E is the electric field strength, B is the magnetic
flux density, H is the magnetic field intensity, and are
magnetic permeability, electric permissivity and
conductivity of the material, respectively.
Maxwell’s first equation is Faraday’s law of induction,
which states that a time variation of flux density is
accompanied by the curl of the electrical field.
Maxwell’s second equation refers to Ampere’s law,
whose integral form states that the magnetic field over a
closed path is equal to the enclosed current.
Equations (6.377) and (6.378) are known respectively as
Gauss’ magnetic law and Gauss’ electric law, while eq.
(6.379) is the constitutive relation for a simple medium.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
31
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
For the microwave thawing process, the enthalpy formulation
including a heat generation term is given by
∂h
= ∇ ⋅ (k ∇ T ) + q′′′
∂t
where h is enthalpy and k is the thermal conductivity.
The heat generation term accounts for the conversion of microwave
energy to heat energy.
Its relationship to the electrical field intensity E at that location can
be derived from eqs. (6.375) – (6.379):
q′′′ = 2π f ε 0ε ′′ E
(6.380)
2
(6.381)
where the magnetic losses in the food material have been ignored.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
32
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The microwave frequency f is 2450 MHz for most
domestic ovens and 915 MHz for some industrial
equipment.
ε0 is the dielectric constant of free space, and ε″ is the
loss factor for the dielectric being heated.
The basic assumptions concerning the heat generation
term include the following:
a)
b)
c)
d)
The microwaves are planar and propagate perpendicularly to
the material.
The microwave field at the material surface is uniform.
Microwave energy entering from different sides is considered
separately, and decreases exponentially from the surface into
the material.
The total amount of heat absorbed by a sample during a
heating cycle is constant.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
33
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Generally, it is difficult to either measure or calculate the strength of
the electrical field inside the material from Maxwell’s equations, so
an exponential variation in the field intensity from the surface of the
material into its interior is generally assumed (Datta, 1990):
2
Ex = E02 exp(− 2α x)
Substituting eq. (6.382) into eq. (6.831), we have
q′′′x = q′′′x 0 exp(− 2α x)
(6.382)
(6.383)
where q′′′x 0 is the rate of heat generation corresponding to an electric
field E0 at the surface of the material, and x is the distance from the
surface into the material.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
34
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The heat generation term can be expressed in general
as
q′′′ = q′′′x 0 exp(− 2α x) + q′′′y 0 exp(− 2α y ) + q′′′z 0 exp(− 2α z )
(6.384)
where q′′′x 0 , q′′′y 0 and q′′′z 0 are the rates of heat generation
corresponding to an electric field E0 at the surfaces of
the material in the different coordinate directions.
The attenuation coefficient α is calculated from the
dielectric constant ε′, the dielectric loss factor ε″, and the
wavelength λ0 in vacuum, which determines the energy
distribution within the material:
2π ε ′{[1 + (ε ′′ / ε ′ ) 2 ]1/ 2 − 1}
α =
(6.385)
λ0
2
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
35
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
In the microwave thawing experiments conducted by Zeng (1991),
frozen cylinders (D×H = 2.0 cm × 2.0 cm) of Tylose were thawed.
Aluminum foil covered both ends, so microwave energy entered
only in the radial direction.
Symmetry about the two central axes of the cylinder is assumed.
Figure 6.38 shows the comparison of the predicted and
experimental temperature histories for a D×H = 2.0 cm × 2.0 cm
cylinder (Case 3a).
For the time interval of 0 ≤ t < 25 s , predicted temperature curve is
rather flat since there is no microwave radiation during “off'” period.
For 25 s ≤ t < 30 s , the microwave oven radiated the Tylose sample.
The incident microwave energy was dissipated into heat energy
within the sample, which caused the sharp temperature rise.
For 30 s ≤ t < 170 s , the temperature history indicates that mushy
region phase change was occurring.
At t ≥ 170 s, the thawing process was completed, and the
temperature rose sharply.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
36
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
history during microwave thawing of a Tylose cylinder (P=64 W).
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
37
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9.5 Laser Drilling
Melting and vaporization occur
Assuming vaporization has started and the liquid-vapor
interface is formed, the geometric shape of the liquidvapor interface and solid-liquid interface are respectively
expressed as
f1 ( z , r , t ) = z − s1 (r , t ) = 0
(6.386)
f 2 ( z , r , t ) = z − s2 ( r , t ) = 0
(6.387)
The temperatures at the two interfaces satisfy
T = Tsat , z = s1 (r , t )
(6.388)
(6.389)
f 2 ( z , r , t ) = z − s2 ( r , t ) = 0
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
38
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.39 Physical model of laser drilling process.
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
39
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The energy balance at the liquid-vapor and solid-liquid interfaces
can be used to obtain the locations of the two interfaces
2
r
−
T
−
T
∂ s1
∂
s
m
1
(6.390)
ρ hl′ v
= α a I 0 e R − kl sat
1
+
, z = s1 (r , t )
2
2
∂t
s2 − s1
T − Tm
∂ s ∂T
ρ hsl 2 = ks s + kl sat
1+
∂t ∂ z
s2 − s1
(6.391)
Ganesh et al derived an equation of saturation temperature by a
using gas dynamic model
hlv 1
∂ Tl
γ +1
1
(6.392)
γ Rg Tsat I abs − kl
−
= p0 exp
γ hlv
∂ r
2
∂ s2
, z = s2 (r , t )
∂ r
∂ n1
Rg Tsat ,0
Tsat
The average material removal rate in the laser drilling process,
which was obtained by the following integration
2π ρ ∞
(6.393)
MR =
s ( r , t ) rdr
tp
∫
0
1
p
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
40
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
6.9.6 Selective Last Sintering (SLS) of Metal
Powders
SLS of metal powder involves fabrication of near full
density objects from powder via melting induced by a
directed laser beam (generally CO2 or YAG) and
resolidification
In order to overcome balling phenomena caused by
surface tension, a powder mixture containing two
powders with significantly different melting points can be
used in the SLS process
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
41
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.41 Physical model of 3-D sintering of two-component metal powder
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
42
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
The liquid velocity, vl, must satisfy the continuity
equation
∂ϕ l
&0
(6.394)
The solid low melting point powder vanishes at the same
rate
∂ ϕ s ∂ (ϕ s ws )
+
= − Φ& 0L
(6.395)
∂t
∂z
The continuity equation for the high melting point
∂ ϕ H ∂ (ϕ H ws )
material is
+
= 0
(6.396)
∂t
∂z
The solid velocity can be determined by integrating eq.
(3)
z> s
0
(6.367)
ws = ϕ s ,i ∂ s
∂t
+ ∇ ⋅ (ϕ l v l ) = Φ
1− ε ∂ t
L
z< s
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
43
6.9 Applications of SolidLiquid Phase Change
The liquid flow occurs in three directions, and the
velocities can be determined using the Darcy's law
KK rl
(6.368)
v − wk=
(∇ p + ρ gk )
l
Chapter 6: Melting and Solidification
s
ϕ lµ
c
l
The temperature transforming model using a fixed grid
method is employed to describe melting and
resolidification in the powder bed and the energy
equation is
∂
{ ϕ ρ c + (ϕ + ϕ ) ρ c T } + ∇ ⋅ (ϕ v ρ c T ) (6.369)
H H pH
l
s
L pL
l l L
∂t
∂
ws (ϕ H ρ H c pH + ϕ s ρ L c pL )T = ∇ ⋅ (k ∇ T )
+
∂z
∂
∂
− ρ L [ (ϕ l + ϕ s )b ] + ∇ ⋅ (ϕ l v l b) +
ϕ
w
b
( s s )
∂
t
∂
z
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
pL
44
6.9 Applications of SolidLiquid Phase Change
Chapter 6: Melting and Solidification
Figure 6.42 Comparison of cross-section area obtained by numerical
simulation and experiment (Ni=0.0749, Ub=0.124)
Transport Phenomena in Multiphase Systems by A. Faghri & Y. Zhang
45