* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Organic Chemistry
Survey
Document related concepts
Transcript
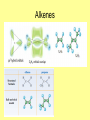
Organic Chemistry Carbon • Has 4 valence electrons • Can have sp3 hybridization where substituted groups are at 109.5o to one another. • Other hybridizations are possible. • Usually shares electrons • Can easily bond to itself and many other elements • Can make straight chains of carbons or branched chains • Can form single, double, and triple bonds to itself and to some selective other atoms • Can polymerize Alkanes • • • • Contain only carbon and hydrogen Have only single bonds. Tetrahedral geometry Characteristic reactions – Halogenation • R-H + X2 R-X + HX • Chlorine >Bromine • Tertiary>Secondary>Primary – Combustion • R-H + O2 CO2 + H2O – Pyrolysis(cracking) • Alkane H2 + smaller alkanes + alkenes Alkanes • Nomenclature – ending is ane. – – – – – – – – – – Meth Eth Prop But Pent Hex Hept Oct Non Dec Alkyl Groups Alkenes • Contain only carbon and hydrogen • Have one double bond between two carbons. • The double bond makes alkenes unsaturated with respect to hydrogen. • Trigonal planar geometry • If there are two or three double bonds – Alkadiene – Alkatriene • Characteristic reactions – Addition – C=C + Y-Z C-C Y Z Alkenes Alkenes • Structural isomers are those which have the same chemical formula but differ in how the atoms are attached to one another. • A cis-isomer has the two substituted groups on the same side of the double bond. • A trans-isomer has the two • substituted groups on • opposite sides of the double • bond. Alkenes • Characteristic Reactions – Addition of hydrogen – Addition of halogens – Addition of hydrogen halides – Addition of sulfuric acid – Addition of water Alkenes • Nomenclature – end with ene – Eth – Prop – But – Pent – Hex – Hept – Oct – Non – Dec Alkynes • • • • Contain only carbon and hydrogen Contain one triple bond between carbons Linear geometry Characteristic reactions of alkynes – Addition of hydrogen – Addition of halogens – Addition of hydrogen halides – Addition of water Alkynes • Nomenclature – end with yne Functional Groups • Hydrocarbons may have functional groups substituted for hydrogens. • Such substitutions give carbon the capacity to form many, varied compounds with different reactivities from the original hydrocarbon. • The possibilities are nearly endless. Alkyl Halides • RX – Give the number of the carbon where the halide(s) appear. – Give the name of the halide (chloro, bromo, iodo) – Write the name of the longest chain of carbons. – C5H10Cl2 dichloropentane (must have structure to determine the numbers of carbons that are substituted) Alcohols • ROH – Give the name of the longest chain of carbons and end with –ol. – Primary – carbon with OH is connected to one other carbon – Secondary – carbon with OH is connected to two other carbons – Tertiary – carbon with OH is connected to three other carbons – C5H11OH – pentyl alcohol or pentanol(structure needed to determine number) Aldehydes • RCHO – Write the name of the longest carbon chain and add -al for the ending. – Remember that the functional group here must be on the last carbon in the molecule. – C5H10O - pentanal Ketones • RCOR` – Write the name of the longest carbon chain and end with -one. – Remember that the functional group, the carbonyl (C=O) cannot be on the end of the molecule. – Example Carboxylic Acids • RCOOH – Write the name of the longest carbon chain and end with –oic acid. – Remember that the functional group (COOH) must be on the end of the molecule. – These are organic acids and they contain one ionizable hydrogen. – Example Esters • RCOOR` • Write the name of the group (R`) and end it with –yl. • Then write the name of the R-C group and end it with –oate. • Example Ethers • ROR` • Write the name of one of the R groups and then the name of the other one in alphabetical order. • End with –ether. • Example Cyclic compounds • • • • Cycloalkanes Cycloalkenes Cycloalkynes Example Aromatic Compounds • These must contain a benzene ring. • Benzene is C6H6 and may be indicated in several different ways. • Positions on the ring may be indicated by ortho(1,2 positioning), meta(1,3 positioning), and para(1,4 positioning) prefixes. • Examples Amines • RNH2 – primary amine • RNR` - secondary amine H • RNR` - tertiary amine R • These are organic bases. • They will combine with carboxylic acids to make amino acids, the building blocks of proteins. • Examples Amides • RCONH2 • Write the name of the carbon chain and end with amide. • Examples Thiols • RSH • May be primary, secondary and tertiary. • Analogs of alcohols so they will do the same reactions. • Name the longest carbon chain and maintain the ending. • Add the word thiol to it.