* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pharmacology for Nurse Prescribing
Survey
Document related concepts
Transcript
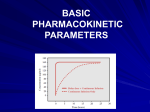
Principles of Drug Dosage, Formulation and Routes of Administration Principles of Drug Dosage All drugs are potentially toxic depending on dose: the aim is to produce a plasma concentration which is effective but not toxic Minimum Effective Concentration The minimum plasma concentration at which a therapeutic response (pharmacodynamic effect) is obtained Maximum Recommended Concentration (or Minimum Toxic Concentration) The maximum effective plasma concentration, above which toxic side effects occur Pharmacokinetic Concepts Pharmacokinetics encompasses the absorption, distribution, metabolism, and excretion of a drug Pharmacokinetic parameters (ADME) usually determined in volunteer studies in normal subjects The following concepts assist prescribers in making therapeutic decisions; as before you are not expected to be an expert but a general understanding will assist in prescribing decisions Pharmacokinetic Concepts We will consider the following pharmacokinetic concepts 1. 2. 3. 4. Bioavailability Volume of Distribution Half-life Clearance Pharmacokinetic Concept 1: Bioavailability The proportion of an administered dose of a drug which reaches the circulation intact For an IV administered drug, the proportion is 100%, i.e. a factor of 1 If the bioavailability of the oral form of the same drug is 0.5, then only 50% of the original dose has reached the circulation intact Bioavailability contd. Propranolol has an oral bioavailability of about 0.05 because of ‘first-pass’ metabolism (100mg oral = 5mg IV) Digoxin oral bioavailability 0.7, morphine approx. 0.3 So for morphine a 10mg iv dose might be equivalent to approx. 30mg oral Pharmacokinetics Concept 2: Volume of Distribution (Vd) Reflects the extent of drug distribution Each drug has a unique volume of distribution Not a real (or physiological) volume, but an ‘apparent’ volume based on plasma concentration following a known dose of the drug In general, high Vd reflects wide distribution to the various organs and tissues, low Vd means that drug stays in the plasma and ECF Volume of Distribution contd. Warfarin (Vd 10L) binds tightly to plasma protein and remains in the bloodstream; Gentamicin (Vd 15L) very water soluble Chloroquine (Vd 13,000L) distributes out of the plasma and binds tightly to cells in the retina Note that 13,000L is an “apparent volume” or it would be a very big person! Pharmacokinetic Concepts 3: Elimination Half-life (t½) Half-life is associated with both accumulation and elimination of drugs It is the time taken for the concentration of the drug in the plasma to increase (accumulation) or decrease (elimination) by half (50%) It is dependent on volume of distribution (Vd) and clearance (Cl) Half-life (t½) contd. Half life determines the time to reach constant effective concentrations in the plasma and the appropriate dosing interval to maintain that concentration For drugs with a short half-life e.g. ferrous sulfate dosing will need to be three or four times a day (unless in a sustained release formulation – see later); for drugs with a long half-life e.g. thyroxine, dosing is once daily Pharmacokinetic Concept 4: Clearance The clearance (Cl) of the drug measures the ability of the body to eliminate the drug It is expressed as volume/unit of time (e.g. mL/min) and represents the volume of blood completely cleared of the drug per unit time Major routes of elimination are the kidney (renal clearance) and liver (hepatic clearance), and others such as lung and sweat (minor sites) Clearance contd. Clearance is a very important parameter in the determination of maintenance doses Clearance of many drugs is affected by organ function, especially the kidney Kidney function is estimated using the glomerular filtration rate (GFR), expressed as mL/min Creatinine clearance (ClCr) is the most common estimate used for GFR Clearance contd. Creatinine is used to estimate renal function: it is a metabolite produced at a relatively constant rate (related to muscle mass), completely filtered by the kidney, and not reabsorbed from the nephron If we measure appearance of creatinine in the urine over a given period of time (e.g. 24h) we can estimate ClCr and GFR Usually difficult to get a 24h urine sample, so we can use equations or nomograms to get an estimate of ClCr Clearance cont. Creatinine clearance often derived using serum creatinine levels by nomograms or by the Cockcroft and Gault equation: Estimated ClCr = (140 – age in years) x (body wt in kg) x (1.04 females or 1.23 males) (mL/Min) Serum Creatinine (micromol/L) From the equation you will note that the estimate is based on the patient’s age, weight, gender and their serum creatinine levels Clearance cont. Normal ClCr is above 100 mL/min Dose of many drugs may have to be adjusted according to ClCr or other markers of renal function Reference books have dose adjustments based on ClCr, e.g. BNF Classification of Renal Impairment Level of impairment GFR (estimated from CrCl) (mL/min) Mild 50-20 Moderate 20-10 Severe <10 End Stage <5 Adapted from Clinical Pharmacy and Therapeutics. 2nd ed. Walker and Edwards. Formulation A novel active substance is of no practical use unless it can be formulated into a dosage form that allows it to be used in real patients Pharmaceutical companies may invest nearly as much in developing the best formulation for a drug as in the original discovery of the molecule (sometimes more) Formulation cont. Very few drugs administered as pure substance Non-medicinal substances are used to enhance characteristics such as appearance, stability, solubility, taste E.g. 100 mg ascorbic acid tablet contains 100mg of active, but the tablet itself is much heavier Design and formulation needs to take into account physical, chemical, and biological nature of all ingredients Importance of Formulation To protect drug from atmospheric variations such as humidity e.g. polished coating on tablets, sealing of ampoules To protect oral doses from destruction due to gastric acidity e.g. enteric coating To conceal bitter/salty or offensive tastes or odours e.g. antibiotics, theophylline To suspend drugs that are insoluble e.g. paracetamol mixture Importance of Formulation contd. To provide clear liquid dose forms e.g. injectables, syrups To provide rate-controlled drug release (prolonged or extended effect) e.g. Ferro-Gradumet® To provide for drug to be given directly to bloodstream e.g. intravenous injections or infusions Oral Formulations Concentrate on oral because it is the main route of administration Many different presentations e.g. tablets, capsules, suspensions, solutions, mixtures, emulsions, syrups, elixirs, linctuses, powders etc. Capsules and tablets can be formulated to provide either immediate-release or prolongedrelease throughout the g.i. tract ER Preparations contd. A variety of terms are used to describe these formulations e.g sustained release (SR), long-acting (LA), retard release (RETARD), extended release (ER), controlled release (CR), extended release (XR) etc. Generally reserved for drugs with a relatively short halflife where frequent dosing would be required Ferrogradumet® is a sustained release form of ferrous sulfate ER Preparations contd. Advantages include prolongation of drug action, reduction in dosing frequency, reduction of side-effects, improved patient compliance Disadvantages include loss of flexibility in dosing, dose-dumping in some cases, technology failure (more in early days), may be expensive, may be problematic in poisoning cases Different Iron Salts Oral iron preparations containing different iron salts are available Each has a slightly different side effect profile, elemental iron content and cost Iron salt Amount Iron Content Ferrous fumarate 200 mg 65 mg Ferrous gluconate 300 mg 35 mg Ferrous sulfate 300 mg 60 mg * Adapted from British National Formulary (BNF) 54th edition Why should we know about routes of administration? Other than if administered iv or intended for local effects, drugs must enter the circulation before distribution to intended sites of action So for many drugs the route chosen is about controlling or overcoming absorption barriers Choosing the ‘optimal’ route of administration is a very important decision in terms of overall therapeutics – onset of action, dose, toxicity etc. Principal Routes of Drug Administration Injection (parenteral) – iv, im, sc etc. Oral (includes enteral feeding) – absorption principally from small intestine Buccal/sublingual Rectal Inhaled Transdermal Topical (includes skin, eyes, ears etc.) Injection Routes General: - Intravenous - Intramuscular - Subcutaneous Specialised: - Intra-articular - Epidural - Intrathecal - etc. Intravenous Route Advantages include: no barrier to absorption; rapid onset; can use loading/bolus doses; can use intermittent infusion or continuous infusion; rapid cessation of action Disadvantages include: patient may need to be hospitalised or have specialist healthcare worker to administer; cost; possibility of infection; inconvenient/unpleasant for patient; mostly restricted to water soluble drugs Numerous examples: gentamicin; morphine; heparin; diazepam; iron sucrose Intramuscular Route Advantages include no need to hospitalise; patient can self-administer; can use depot injections; suitable for water-insoluble drugs Disadvantages include painful; slower distribution; slower onset of action; variable absorption; may need higher dose; smaller volumes than iv Examples: Iron Polymaltose Complex; Depo-Medrol ® (methylprednisolone); Modecate® (fluphenazine) Subcutaneous Route Advantages include patient can selfadminister, suitable for implants; pain-relief infusion devices can be used Disadvantages include slower onset of action; irritation at the site; small volumes; small doses Examples: insulin, low molecular wt heparin Oral Route Most frequent and convenient route of administration Most medicines are administered by this route – – – – – Tablets Solution Suspension Powder Capsule Oral Route Tablets and capsules can be formulated for ‘immediate’ release or ‘extended’ release in g.i. tract Tablets may be effervescent Main site of absorption is small intestine Oral Route contd. Advantages include patient-controlled; convenient/portable; comparatively low cost; a variety of techniques to provide extended release Disadvantages include bioavailability concerns; first-pass metabolism; relatively large doses required; drug/food interactions; time lapse to effect; compliance; dose frequency Oral Route contd. Numerous examples: Multivitamins (powder, tablets, liquids) Calogen (emulsion) Paracetamol (suspension, tablets, capsules) Morphine (solution, extended release tablets) Phenoxymethylpenicillin (suspension, tablets) Dosing Considerations – Short Bowel Consider a patient with a short bowel due to surgery or disease Which region of bowel is functioning? Where is the prescribed drug absorbed? E.g. Ferrogradumet® may not be suitable for a patient with a short bowel, as the transit time is too short for all the drug to release and be absorbed. An immediate release product may be more appropriate.