* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Cardiac Rupture Complicating Acute Myocardial Infarction in the
History of invasive and interventional cardiology wikipedia , lookup
Remote ischemic conditioning wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Jatene procedure wikipedia , lookup
Cardiac surgery wikipedia , lookup
Cardiac contractility modulation wikipedia , lookup
Coronary artery disease wikipedia , lookup
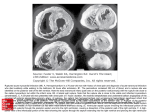
Cardiac Rupture Complicating Acute Myocardial Infarction in the Direct Percutaneous Coronary Intervention Reperfusion Era* Hon-Kan Yip, MD; Chiung-Jen Wu, MD; Hsueh-Wen Chang, PhD; Chao-Ping Wang, MD; Cheng-I Cheng, MD; Sarah Chua, MD, FCCP; and Mien-Cheng Chen, MD Background: Cardiac rupture, an uncommon yet catastrophic complication after acute myocardial infarction (AMI), has been studied primarily in the prethrombolytic and thrombolytic therapy eras but not in the direct percutaneous coronary intervention (d-PCI) reperfusion therapy era. The aim of this study was to delineate the incidence, potential risks, timing of occurrence, clinical features, and outcomes of cardiac rupture complicating AMI after d-PCI. Methods and results: Between May 1993 and July 2002, a total of 1,250 patients with AMI underwent d-PCI in our hospital. Of these 1,250 patients studied, 12 patients (0.96%) had cardiac rupture (ventricular septal defect [VSD], three patients; left ventricular [LV] free wall rupture, nine patients] after d-PCI, with a mean (ⴞ SD) time of occurrence of 52.3 ⴞ 36.2 h. Three patients with VSD had an insidious presentation, and two of these patients (66.6%) survived after surgical intervention. However, nine patients with LV free wall rupture always presented with sudden and unanticipated hemodynamic collapse. Cardiopulmonary resuscitation was uniformly unsuccessful in patients with LV free wall rupture, and all patients died as a result of this complication within minutes of its onset. The 30-day mortality rate was significantly higher in patients with cardiac rupture than in patients without this complication (83.3% vs 8.2%, respectively; p < 0.001). Univariate analysis demonstrated that the left anterior descending artery was the most likely to be totally occluded in patients who had developed cardiac rupture (100% vs 66.4%, respectively; p ⴝ 0.033). Multiple stepwise logistic regression analysis demonstrated that the most significant factors associated with cardiac rupture were advanced age, female gender, and lower body mass index (BMI; all p < 0.05), whereas early reperfusion with d-PCI was an independent determinant of preventing this complication (p < 0.0001). Conclusion: Compared with the prethrombolytic era, our study showed that d-PCI had a favorable impact on reducing the incidence of cardiac rupture after AMI. Old age, female gender, lower BMI, and longer time to reperfusion carried a substantially increased risk of cardiac rupture after patients experienced AMIs. Early successful d-PCI was the most powerful determinant of the avoidance of this catastrophic complication after AMI. (CHEST 2003; 124:565–571) Key words: acute myocardial infarction; cardiac rupture; direct percutaneous coronary intervention Abbreviations: AMI ⫽ acute myocardial infarction; BMI ⫽ body mass index; d-PCI ⫽ direct percutaneous coronary intervention; IRA ⫽ infarct-related artery; LV ⫽ left ventricle ventricular; TIMI ⫽ Thrombolysis in Myocardial Infarction; VSD ⫽ ventricular septal defect upture of the myocardium after acute myocarR dial infarction (AMI) may involve the free wall of the left ventricle (LV), the interventricular septum, or the papillary muscles.1–3 While LV free wall rupture and ventricular septal defect (VSD) are uncommon mechanical complications after AMI, *From the Division of Cardiology (Drs. Yip, Wu, Wang, Cheng, Chua, and Chen), Chang Gung Memorial Hospital, Kaohsiung, Taiwan, Republic of China; and the Department of Biological Sciences (Dr. Chang), National Sun Yat-Sen University, Kaohsiung, Taiwan, Republic of China Manuscript received August 27, 2002; revision accepted December 12, 2002. Reproduction of this article is prohibited without written permission from the American College of Chest Physicians (e-mail: [email protected]). Correspondence to: Mien-Cheng Chen, MD, Division of Cardiology, Department of Internal Medicine, Chang Gung Memorial Hospital, Kaohsiung 123, Ta Pei Rd, Niao Sung Hsiang, Kaohsiung Hsien, 83301, Taiwan, Republic of China; e-mail: chenmien@ kinghenry.com.tw www.chestjournal.org Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 CHEST / 124 / 2 / AUGUST, 2003 565 they carry an extremely high mortality rate.1– 4 The incidence,2,4 – 6 timing of occurrence,7,8 prognostic factors,2 and clinical features9,10 and outcomes1– 4,11,12 of AMI complicated by VSD and LV free wall rupture in both the prethrombolytic and thrombolytic therapy eras have been debated extensive. However, there are no available data with regard to the incidence, clinical features, and outcomes of these complications in patients with AMI undergoing direct percutaneous coronary intervention (d-PCI). Furthermore, previous studies11,12 have demonstrated that although thrombolytic therapy can reduce the incidence of cardiac rupture, this therapeutic management for patients with AMI also may accelerate early cardiac rupture.2,12 Whether this paradoxical effect of thrombolytic therapy also occurs in the present d-PCI reperfusion era remains unknown. Therefore, the aim of this study was to investigate the incidence, associated potential risk factors, timing of occurrence, and clinical outcomes of this complication in patients with AMI who have receiving d-PCI therapy, and the impact of d-PCI on and the importance of reperfusion time in this complication. Materials and Methods Patient Population In our hospital, there were only a small number of patients with AMI who either refused d-PCI or were treated with lytic therapy. Most of the patients with AMI underwent d-PCI after informed consent was obtained. For the purpose of the study, all patients who underwent d-PCI were prospectively identified and entered into a computerized database. Between May 1993 and July 2002, d-PCI was performed in 1,250 patients of any age who had presented with AMI of ⬍ 12 h duration (or had experienced cardiogenic shock within 18 h) in our hospital. Of these 1,250 patients, 12 (0.96%) had cardiac rupture (including 3 patients with VSD and 9 patients with LV free wall rapture) that had occurred after receiving d-PCI. These 12 patients constituted our study population (group 1). The remaining 1,238 patients served as the control group (group 2). Procedure and Protocol The procedure and protocol have been described previously in detail.13,14 Before stents were available in our country, primary balloon angioplasty was performed in these patients, however, after stents were available in our country, stent implantation was strongly encouraged unless the infarct-related artery (IRA) had heavy calcification, a reference lumen diameter of ⬍ 2.5 mm, or a stent-like formation on the treatment site after coronary angioplasty. Left ventriculograms, which were immediately performed after angioplasty, were recorded for 30° right anterior oblique and 60° left anterior oblique views. Echocardiography was routinely performed in each patient either before or after d-PCI. Platelet glycoprotein IIb/IIIa receptor antagonists have been available in our country since August 2000. In our hospital, all patients with AMIs are considered eligible for therapy with glycoprotein IIb/IIIa on presentation in the emergency depart- ment after informed consent is obtained, unless there are contraindications. As a result of our government medical insurance policy, only 8 patients (including 1 patient with LV free wall rupture) received abciximab therapy (a loading dose of 0.25 mg/kg body weight, followed by a maintenance infusion of 0.1 mg/min for 18 to 24 h), and 300 patients (including 2 patients with LV free wall rupture) received tirofiban therapy (a loading dose of 20 g/kg body weight, followed by a maintenance infusion of 0.15 g/min for 18 to 24 h). Continuous heparin infusion for a further 24 to 48 h was administered only to patients who received balloon angioplasty. Patients were treated with ticlopidine for 2 weeks if stenting was performed. Aspirin (100 mg po once a day) was administered to each patient indefinitely. All other medications, including nitrates, beta-blockers, diuretics, and angiotensin-converting enzyme inhibitors were used as needed in both groups. Definitions AMI was defined as the following: (1) typical chest pain lasting for ⬎ 30 min with ST-segment elevation of ⬎ 1 mm in two consecutive precordial or inferior leads and (2) typical chest pain lasting for ⬎ 30 min with a new onset of complete left bundle branch block. Procedural success was defined as a reduction in residual stenosis of ⬍ 50% by balloon angioplasty or successful stent deployment at the desired position with a residual stenosis of ⬍ 20% followed by Thrombolysis in Myocardial Infarction (TIMI)15 grade 3 flow in the IRA. Body mass index (BMI) was defined as the weight (in kilograms) divided by the square of the height (in meters). VSD was first suspected by physical examination and subsequently was confirmed by echocardiography, a significant step-up in oxygen saturation between the right atrium and the pulmonary artery using a Swan-Ganz catheter, and operative findings. LV free wall rupture was suspected by a patient’s sudden hemodynamic collapse associated with the patient’s loss of consciousness and electromechanical dissociation without ECG evidence of malignant ventricular tachyarrhythmias. A diagnosis subsequently was made by echocardiographic findings of a new accumulated pericardial effusion and further was confirmed by pericardiocentesis via an apical approach method. Data Collection Detailed in-hospital and follow-up data including age, sex, coronary risk factors, Killip score on hospital admission, reperfusion time, pre-TIMI and post-TIMI flow grades, angiographic results, number of diseased vessels and coronary aneurysms, and number of in-hospital adverse events were obtained. These data were collected prospectively and were entered into a computerized database. Statistical Analysis Data were expressed as the mean ⫾ SD. Continuous variables were compared using the Wilcoxon rank sum test. Categoric variables were compared using the 2 test or Fischer exact test. Stepwise logistic regression analysis was used to determine the independent determinants of cardiac ruptures after d-PCI. Statistical analysis was performed using a statistical software package (SAS for Windows, version 6.12; SAS Institute; Cary, NC). A probability value of ⬍ 0.05 was considered to be statistically significant. 566 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 Clinical Investigations Results Baseline Clinical Characteristics of the Patients Relevant patient baseline characteristics are summarized in Tables 1. There were no significant differences in terms of the presence of diabetes mellitus or hypercholesterolemia, previous myocardial infarction, infarction locations, and the incidences of cardiogenic or noncardiogenic shock between patients with cardiac rupture and those without. However, patients with cardiac rupture had significantly higher incidences of hypertension and current smoking than did patients without this complication. Furthermore, patients with cardiac rupture were significantly older and thinner than were patients without this complication. By univariate logistic regression analysis, old age was significantly related to increased incidence of cardiac rupture (odds ratio, 1.11; 95% confidence interval, 1.04 to 1.18; p ⫽ 0.0007). Moreover, the incidence of female gender was significantly higher in patients with cardiac rupture than in patients without it. In this study, female patients were significantly older than male patients (67.1 ⫾ 11.7 vs 60.8 ⫾ 11.9, respectively; p ⫽ 0.0001). In addition, in the 12 patients with cardiac rupture, female patients were also older than male patients, although the difference did not reach statistical significance as a result of a type-2 error (75.1 ⫾ 7.7 vs 70.5 ⫾ 3.5, respectively; p ⫽ 0.286). Table 1—Baseline Characteristics Between Patients With and Without Cardiac Rupture* Variables Age, yr Female sex Hypertension Smoking Diabetes mellitus Hypercholesterolemia Previous MI BMI Preinfarction angina Infarction location by ECG Anterior Inferior Lateral Non-Q wave Killip classification Cardiogenic shock Noncardiogenic shock Cardiac Rupture (n ⫽ 12) Noncardiac Rupture (n ⫽ 1,238) 73.6 ⫾ 6.8 66.7 (8/12) 83.3 (10) 25 (3) 25 (3) 41.7 (5) 0 (0) 22.3 ⫾ 4.1 16.7 (2) 61.7 ⫾ 11.9 15.7 (194) 49.0 (607) 55.7 (690) 25.2 (312) 43.4 (537) 11.1 (138) 25.5 ⫾ 4.1 31.7 (393) 83.3 (10) 16.7 (2) 0 (0) 0 (0) 56.9 (705) 39.4 (488) 1.9 (23) 1.8 (22) 16.7 (2) 83.3 (10) 12.7 (157) 87.3 (1081) p Value 0.0001 ⬍ 0.0001 0.018 0.033 1.000 0.905 0.382 0.006 0.359 0.406 0.657 *Values given as mean ⫾ SD or % (No. of patients), unless otherwise indicated. MI ⫽ myocardial infarction. Timing of Occurrence, Clinical Presentation, and Outcomes of Cardiac Rupture Twelve of the 1,250 patients (0.96%) had cardiac rupture, including 3 with VSD and 9 with LV free wall rupture. However, neither pseudoaneurysm nor incomplete rupture of the LV free wall was found in these patients. Cardiac rupture developed after dPCI in a mean time of 52.3 ⫾ 36.2 h (range, 4.8 h to 5 days). Clinical symptoms and signs in patients with VSD mostly showed an insidious presentation, and progressive dyspnea, worsening congestive heart failure, and pansystolic heart murmur during physical examination were the usual initial presentations. In contrast, patients with LV free wall rupture always presented with precipitous and unanticipated hemodynamic collapse. ECG monitoring showed sinus tachycardia, sinus bradycardia, junctional escape rhythm, or standstill without evidence of ventricular tachycardia/fibrillation. Echocardiograms showed pericardial effusion (Fig 1). Pericardiocentesis was performed in five patients and showed clotted blood in all five. Cardiopulmonary resuscitation was uniformly unsuccessful in patients with LV free wall rupture, and all patients died as a result of this complication within minutes after its onset. An operation was performed in the three patients who had acquired VSD. One patient died in the hospital due to multiorgan failure, and the other two patients were discharged from the hospital uneventfully and have survived to the present. Angiographic Findings, Angioplasty Results, Reperfusion Time, and 30-Day Mortality Rate of Patients Relevant angiographic results, the time to reperfusion, and clinical outcome are summarized in Table 2. There were no significant differences in terms of IRA, number of vessels affected by the disease, intercoronary collaterals, pre-TIMI and post-TIMI flow grades, or methods of reperfusion between patients with and without cardiac rupture. However, the incidence of a totally occluded left anterior descending artery (Fig 2) was significantly higher in patients with cardiac rupture than in patients without it (100% [9 of 9 patients] vs 66.4% [463 of 697 patients]; p ⫽ 0.033). Furthermore, the mean time to reperfusion was significantly longer, and the mortality rate was significantly higher in patients with cardiac rupture than in patients without it. Independent Predictors of Cardiac Rupture Independent determinants of cardiac rupture are summarized in Table 3. Multiple stepwise logistic regression analysis demonstrated that only old age, www.chestjournal.org Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 CHEST / 124 / 2 / AUGUST, 2003 567 Figure 1. Left, A: echocardiography (M-mode) was performed after primary stenting, and there was no pericardial effusion. Middle, B (apical four-chamber view), and Right, C (M-mode): 3 days later, after primary stenting, sudden hemodynamic collapse occurred in the patient. Echocardiography was performed, and the result demonstrated that there had been new accumulated pericardial effusion (black arrows). female gender, a lower BMI, and a longer time to reperfusion were significant independent predictors of cardiac rupture. Discussion Incidence of Cardiac Rupture in the d-PCI Reperfusion Era In the present study, we found that the overall incidence of cardiac rupture was 0.96%, including Table 2—Angiographic Features, Angioplasty Results, and Mortality in Patients With and Without Cardiac Rupture* Variables Cardiac Rupture (n ⫽ 12) Noncardiac Rupture (n ⫽ 1,238) 0.24% for VSD and 0.72% for LV free wall rupture. To the best of our knowledge, this is the first study to investigate the incidence, timing of occurrence, clinical features, and outcomes, as well as the predictors of myocardial rupture in patients with AMI undergoing d-PCI. The incidence of cardiac rupture was previously reported to be 4% in the prethrombolytic therapy era5,6 and ⬍ 1.0% in the thrombolytic therapy era.2,12 In addition, the incidence of LV free wall rupture was reported to be 8 to 10 times more frequent than that of VSD and the rupture of the papillary muscle.16 Our findings are consistent with those of previous thrombolytic reperfusion studies2,7,12 and further confirm the concept that early successful reperfusion either by thrombolysis or d-PCI may reduce the incidence of cardiac rupture after AMI. p Value IRA 0.645 LAD 83.3 (10) 56.3 (697) LCX 0 (0) 7.3 (90) RCA 16.7 (2) 34.5 (427) LM 0 (0) 1.9 (24) Multivessel disease 58.0 (7) 57.0 (706) 0.928 (ⱖ 2 vessels) Present intercoronary 8.3 (1) 31.4 (389) 0.0705 collaterals Pre-TIMI flow 0.070 TIMI-0 flow 91.7 (11) 65.9 (816) ⱖ TIMI-1 flow 8.3 (1) 34.1 (422) Post-TIMI flow 0.661 TIMI-3 flow 83.3 (10) 84.8 (1,050) TIMI-2 flow 16.7 (2) 10.3 (127) ⱕ TIMI-1 flow 0 (0) 4.9 (61) Method of reperfusion 0.421 Balloon 58.3 (7) 46.7 (578) Stent 41.7 (5) 53.3 (660) Mean time to 553.9 ⫾ 201.4 283.2 ⫾ 153.9 0.0007 reperfusion, min Mortality rate 83.3 (10) 8.2 (102) ⬍ 0.0001 *Values given as mean ⫾ SD or % No. of patients. LAD ⫽ left anterior descending; LCX ⫽ left circumflex; LM ⫽ left main; RCA ⫽ right coronary artery. Timing and Possible Mechanisms of Cardiac Rupture in the d-PCI Reperfusion Era It has been stated7,8 that the peak incidence of cardiac rupture occurs 5 to 7 days after infarction in the prethrombolytic therapy era. To the contrary, most cardiac ruptures that are associated with early thrombolytic therapy occur within 24 h after treatment.2,12,16 These large clinical trials2,12,16 also have demonstrated that although the frequency of cardiac rupture decreases, early thrombolytic therapy (ie, within 6 h) paradoxically accelerates the timing of this complication, and late thrombolytic therapy has been found to increase the risk of cardiac rupture.11 Several possible mechanisms, including extension of myocardial hemorrhage weakening and dissection of the necrotizing zone,12,16 diminishing of the myocardial collagen content,17 and digestion of collagen by collagenases18,19 and plasmin,20 have been suggested. In the present study, several important observations were made. First, the timing of cardiac rupture 568 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 Clinical Investigations Figure 2. Left, A: right coronary angiogram showed chronic total occlusion of the proximal right coronary artery (black arrow head) without intercoronary collaterals to the left anterior descending artery. Middle, B: left coronary angiogram showed total occlusion of the proximal left anterior descending artery (bigger black arrow head) and moderate atherosclerosis of proximal big obtuse marginal branch (smaller black arrow head). Right, C: successful stent implantation in the proximal left anterior descending artery (black arrow heads) with normal coronary blood flow. in our patients varied (range, 4.8 h to 5 days) with a mean time of 52.3 h. The mean time of occurrence of this complication in our patients was earlier than that in the prethrombolytic therapy era7,8 but was later than that in the thrombolytic therapy era,2,16 which suggests that reperfusion therapy by d-PCI does not accelerate the timing of cardiac rupture, as occurred with thrombolytic therapy.2,12,16 Second, our patients with cardiac rupture had significantly longer time to reperfusion than did those without cardiac rupture. This finding suggests that the risk of cardiac rupture after AMI is related directly to the timing of reperfusion. Third, angiographic findings demonstrated that the incidence of a totally occluded left anterior descending artery was significantly higher in patients with cardiac rupture than in patients without it. This finding suggests that a transmural myocardial infarction is more likely to occur in these patients. Relationships Among Age, Gender, BMI, and Cardiac Rupture Advanced age has been found to be an increased risk factor for adverse outcomes after AMI.21 The risks increased proportionally with advancing age, Table 3—Multiple Stepwise Logistic Regression Analysis of Independent Predictors of Cardiac Rupture* Variables OR 95% CI p Value Age Female sex BMI Reperfusion time 1.085 4.854 1.264 0.994 1.008–1.168 1.307–17.857 1.036–1.542 0.992–0.997 0.0272 0.0001 0.0095 0.0001 *OR ⫽ odds ratio; CI ⫽ confidence interval. and primary angioplasty did not alter the relationship between adverse outcomes after AMI and being aged.21 The contributing factors to this relationship included the presence of additional comorbid conditions, more advanced multivessel disease, and a longer time between the onset of symptoms and presentation for evaluation and treatment.21–23 In the present study, old age was an independent predictor of cardiac rupture. Our finding confirmed the strong relationship between advanced age and increased adverse outcomes in the clinical setting of AMI. Morbidity and mortality after AMI have been reported to be higher in women than in men.24 In the present study, we also found that female gender was another independent predictor of cardiac rupture. In the present study, it is interesting to note that women constituted only 16.2% of our patient population. However, it is surprising that ⬎ 66% of patients with cardiac rupture were women. We remain uncertain why women are more likely to experience cardiac rupture after AMI. However, this may be due to older age in female patients and to other fundamental differences in the biology and pathophysiology of AMI between men and women.24 In patients with coronary artery disease who are undergoing PCI, lean patients recently have been reported25 to be at increased risk for in-hospital complications and cardiac death. However, the mechanism by which lean patients have an excess risk for these complications remains unclear.25 In the present study, we also found that patients with cardiac rupture had significantly lower BMI values than those without this complication. Furthermore, multiple stepwise logistic regression analysis demonstrated that BMI was an independent predictor of www.chestjournal.org Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 CHEST / 124 / 2 / AUGUST, 2003 569 cardiac rupture. This may reflex a synergic effect of being female and lean body weight on the predilection of cardiac rupture after AMI. Formidable Challenges for Management of Cardiac Rupture It is estimated that 8 to 17% of all fatal myocardial infarctions are the result of myocardial rupture.26 Despite improvements in medical therapy and in percutaneous and surgical techniques, mortality from this complication remains extremely high.2 These patients usually die immediately, even before a diagnosis can be confirmed.4 In the present study, we found that the mortality rate caused by cardiac rupture was 8.9% (10 of 112 patients) of all fatal myocardial infarctions in our patient population. Our finding is consistent with previous observations.12,26 The overall mortality rate was very high (83.3%) in patients with cardiac rupture. It was disappointing that all of our patients with LV free wall rupture died despite heroic therapeutic bedside management. The primary obstacle in preventing death from LV free wall rupture is the extremely limited time in which surgery can be initiated, as the occurrence of LV free wall rupture is sudden and unanticipated without a constellation of signs or symptoms to indicate impending rupture in these patients, and it is followed by rapid hemodynamic deterioration. There are several limitations to this study. First, the number of patients with cardiac rupture in this study was small, therefore, our results should be viewed as preliminary and need to await confirmation by larger clinical trials. Second, cardiac rupture, pseudoaneurysm, or incomplete rupture could easily be missed since premortem echocardiograms were often unavailable and cultural factors prevent postmortem examination. Therefore, the incidences of these complications could have been underestimated in our study. Third, as the number of patients with cardiac rupture was small in this study, the lack of significant differences in the incidences of previous myocardial infarction, anterior wall infarction, preinfarction angina, presence of collateral circulation, pre-TIMI flow, and method of reperfusion between patients with cardiac rupture and without could have been due to a type-2 error. In conclusion, advanced age, female gender, a lower BMI, and longer time to reperfusion were significant independent predictors of cardiac rupture after AMI, and d-PCI had a favorable impact on reducing the incidence of cardiac rupture. Early successful d-PCI was the most significant independent determinant for preventing this catastrophic complication after AMI. References 1 Cheriex EC, de Swart H, Dijkman LW, et al. Myocardial rupture after myocardial infarction is related to the perfusion status of the infarct-related coronary artery. Am Heart J 1995; 129:644 – 650 2 Crenshaw BS, Granger CB, Birnbaum Y, et al. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. Circulation 2000; 101:27–32 3 Slater J, Brown RJ, Antonelli TA, et al. Cardiogenic shock due to cardiac free-wall rupture or tamponade after acute myocardial infarction: a report from SHOCK trial registry. J Am Coll Cardiol 2000; 36:1117–1122 4 Pohjola-Sintonen S, Muller JE, Stone PH, et al. Ventricular septal and free wall rupture complicating acute myocardial infarction: experience in the multicenter investigation of limitation of infarct size. Am Heart J 1989; 117:809 – 816 5 Sievers J. Cardiac rupture in acute myocardial infarction. Geriatrics 1966; 21:125–130 6 Rasmussen S, Leth A, Kjoller E, et al. Cardiac rupture in acute myocardial infarction. Acta Med Scand 1979; 205:11–16 7 Schuster E, Bulkley B. Expansion of transmural myocardial infarction: a pathophysiologic factor in cardiac rupture. Circulation 1979; 60:1532–1538 8 Bedynek J, Fenoglio J, McAllister H. Rupture of the interventricular septum as a complication of myocardial infarction. Am Heart J 1979; 97:773–781 9 Bates RJ, Beutler S, Resnekov L, et al. Cardiac rupture: challenge in diagnosis and management. Am J Cardiol 1977; 40:429 – 437 10 Oliva PB, Hammill SC, Edwards WD. Cardiac rupture, a clinical predictable complication of acute myocardial infarction: report of 70 cases with clinicopathologic correlations. J Am Coll Cardiol 1993; 22:720 –726 11 Honan MB, Harrell FE, Reimer KA, et al. Cardiac rupture, mortality and the timing of thrombolytic therapy: a metaanalysis. J Am Coll Cardiol 1990; 16:359 –367 12 Becker RC, Gore JM, Lambrew C, et al. A composite view of cardiac rupture in the United States national registry of myocardial infarction. J Am Coll Cardiol 1996; 27:1321–1326 13 Yip HK, Chang HW, Wu CJ, et al. A safe and effective regimen without heparin therapy after successful primary coronary stenting in patients with acute myocardial infarction. Jpn Heart J 2000; 41:697–711 14 Yip HK, Wu CJ, Chang HW, et al. Comparison of impact of primary percutaneous transluminal coronary angioplasty and primary stenting on short-term mortality in patients with cardiogenic shock and evaluation of prognostic determinants. Am J Cardiol 2001; 87:1184 –1188 15 The Thrombolysis in Myocardial Infarction Study Group. The Thrombolysis in Myocardial Infarction (TIMI) trial: phase 1 findings. N Engl J Med 1985; 312:932–936 16 Becker RC, Charlesworch A, Wilcox RG, et al. Cardiac rupture associated with thrombolytic therapy: impact of time to treatment in the late assessment of thrombolytic efficacy (LATE) study. J Am Coll Cardiol 1995; 25:1063–1068 17 Factor SM, Robinson TF, Dominitz R, et al. Alterations of the myocardial skeletal framework in acute myocardial infarction with and without ventricular rupture: a preliminary report. Am J Cardiovasc Pathol 1986; 1:91–97 18 Charney RH, Takahashi S, Zhao M, et al. Collagen loss in the stunned myocardium. Circulation 1992; 85:1483–1490 19 Whittaker P, Boughner DR, Kloner RA. Role of collagen in acute myocardial infarct expansion. Circulation 1991; 84: 2123–2134 20 Peuhkurinen KJ, Risteli L, Melkko JT, et al. Thrombolytic 570 Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 Clinical Investigations therapy with streptokinase stimulates collagen breakdown. Circulation 1991; 83:1969 –1975 21 Holmes DR, White HD, Pieper KS, et al. Effect of age on outcome with primary angioplasty versus thrombolysis. J Am Coll Cardiol 1999; 33:412– 419 22 Fibrinolytic Therapy Trialists (FTT) Collaborative Group. Indications for fibrinolytic therapy in suspected acute myocardial infarction: collaborative overview of early mortality and major morbidity results from all randomized trials of than 1000 patients. Lancet 1994; 343:311–322 23 White HD, Barbash GI, Califf RM, et al. Age and outcome with contemporary thrombolytic therapy: results from GUSTO-I trial. Circulation 1996; 94:1826 –1833 24 Vakili BA, Kaplan RC, Brown DL. Sex-based differences in early mortality of patients undergoing primary angioplasty for first acute myocardial infarction. Circulation 2001; 104:3034 – 3038 25 Gruberg L, Weissman NJ, Waksman R, et al. The impact of obesity on the short-term and long-term outcome after percutaneous coronary intervention: the obesity paradox? J Am Coll Cardiol 2002; 39:578 –584 26 Reddy SG, Roberts WC. Frequency of rupture of the left ventricular free wall or ventricular septum among necropsy cases of fatal acute myocardial infarction since introduction of coronary care units. Am J Cardiol 1989; 63:906 –911 www.chestjournal.org Downloaded From: http://publications.chestnet.org/pdfaccess.ashx?url=/data/journals/chest/21997/ on 05/13/2017 CHEST / 124 / 2 / AUGUST, 2003 571