* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapters 3
Survey
Document related concepts
Transcript
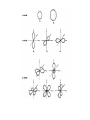
Chapters 3 - 4 How It All Fits Together Developing a model of the atom in order to explain, predict and perform chemical reactions and chemical properties. 3.1 Atom: History (Democritus to Rutherford) • Democritus – indivisible particle (atomos) • Dalton – Atomic Theory (e.g. atoms are rearranged, combined, or separated) • Thomson - Cathode-ray tube (CRT) - discovered electron - plum-pudding model • Rutherford - gold-foil; discovered nucleus; Nuclear Model Historical Development of Atomic Models (side information) • atomic number = #protons • mass number = #protons + #electrons • charge = difference between #protons & #electrons much of what we know (atoms) comes from light • light travels in waves • c=ln • E=hn (energy of a photon; h = Plank’s constant) • E = (h c) / l • Plank – energy comes in quanta (discrete packets of energy; not continuous) (photon ~ light quanta) • Bohr – electrons in fixed orbits – are separated by quanta – experiment: line-emission spectrum of hydrogen atom • dual wave-particle theory explained fixed orbits (analogy: musical scale A, B, ...) • Heisenberg’s uncertainty principle - probable location of an electron • Schrödinger’s wave equation: - () gives the probable location of an electron in x-, y-, z-, and time coordinates. • Quantum numbers (location of electron): - “n” = principle q.n. (principle energy level) - “l” = angular q.n. (shape s-, p-, etc) - “m” = magnetic q.n. (orientation) - “s” = spin (+ ½; ) • Orbital Notation (e.g., sulfur) • Electron Configuration 1s2 2s2 2p6 3s2 3p3 • Noble-Gas Configuration [Ne] 3s2 3p3