* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Development of the Heart
Coronary artery disease wikipedia , lookup
Heart failure wikipedia , lookup
Aortic stenosis wikipedia , lookup
Electrocardiography wikipedia , lookup
Myocardial infarction wikipedia , lookup
Quantium Medical Cardiac Output wikipedia , lookup
Cardiac surgery wikipedia , lookup
Hypertrophic cardiomyopathy wikipedia , lookup
Mitral insufficiency wikipedia , lookup
Congenital heart defect wikipedia , lookup
Arrhythmogenic right ventricular dysplasia wikipedia , lookup
Lutembacher's syndrome wikipedia , lookup
Atrial septal defect wikipedia , lookup
Dextro-Transposition of the great arteries wikipedia , lookup
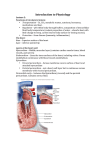
Pamela E. Knapp, Ph.D. Dept. of Anatomy & Neurobiology Embryology DEVELOPMENT OF THE HEART READING: Larsen, 4th Edition, Chapter 12; or, Langman, 8th Edition, pp. 208-239 OBJECTIVES: Following lecture and assigned reading, students should be able to: • Describe formation of paired endocardial tubes that fuse to form a primitive heart tube. • Describe the primitive heart tube, including sinus horns, sinus venosus, primitive atrium and ventricle, bulbus cordis. Include associated arterial structures, truncus arteriosus and aortic sac. • Describe connections between developing heart and inflow (venous structures) and outflow (dorsal aortae) vessels. • Describe development of the pericardial cavity, it’s relationship to the embryonic embryonic coelom, and positional changes driven by embryonic flexion and folding. • Describe development of the atrioventricular canals and role of the endocardial cushions. • Discuss the partitioning of the primitive atrium by septum primum, septum secundum. • Describe the embryological components that contribute to the left and right atria and ventricles. • Be able to describe the role of the foramen ovale in blood flow changes at birth. • Explain the partitioning into right and left ventricles and the separation of both the conus cordis and truncus arteriosus into two separate channels by the aorticopulmonary septum. • Differentiate between the membranous and muscular interventricular septa. • Be able to describe the path of bloodflow through the primitive heart tube, and how it changes with septation of the heart. • Be able to describe (in words and by diagram) the following list of congenital cardiac defects. Be able to discuss the structural abormalities, their result, and any known etiologic factors. 1. Ventricular septal defects 2. Atrial septal defects (differentiate between probe patent foramen ovale and secundum defects) 3. Transposition of the Great Arteries (TGA) 4. Overriding Aorta or Pulmonary Trunk (unequal division of the truncus arteriosus), 5. Tetralogy of Fallot Useful Terminology: Angiogenesis = blood vessel formation Cardiogenesis = heart formation Aortic sac = base of aortic arches Bulbus cordis = primitive heart chamber, it’s distal portion=conus cordis Bulboventricular loop = loop formed when early heart folds upon itself Endocardial cushions = mesenchymal masses that contribute to partitioning of the heart Endocardial tubes = paired tubes that fuse to form the primitive heart tube Primitive heart = tubular structure with grooves delineating chambers, from inflow to outflow: sinus venosus, primitive paired atria, primitive ventricle, bulbus cordus, conus cordis Septum transversum = mesodermal mass (initially in front of pericardial cavity) that separates thoracic and abdominal cavities Septum primum and secundum = portions of the developing interatrial septum Ostium primum and secundum = openings formed by the septum primum and secundum Truncus arteriosus = arterial trunk, opening from bulbus cordis of heart Vitelline = relating to yolk sac Mesenchyme = tissue composed of undifferentiated, multipotential cells. Not equivalent to mesoderm DEVELOPMENT OF THE HEART 01. Extraembryonic blood vessels form during the early part of the third week. Yolk sac (extraembryonic) mesodermal cells aggregate as blood islands and form a network of cords. Central cells of these cords differentiate as blood cells and peripheral cells make up the vessel lining. . Embryonic blood vessels form later during the 3rd week (about two days after first extraembryonic vessels) in the visceral (splanchnic) layer of lateral plate mesoderm. Vessel formation in the embryo is similar to extraembryonic vessel formation, except blood cells do not form from central cells of embryonic vascular cords. 02. The blood islands, labeled here, are located in the floor of celomic cavity (future pericardial cavity). They form from visceral (splanchnic) mesoderm and later will coalesce to form the heart primordia in the form of paired endocardial tubes. Mesoderm that is “in front of” the primitive pericardial cavity (connecting mesoderm of amnion and yolk sac) is called the transverse septum. 03. Looking down on the embryonic disc, and through ectoderm and somatic mesoderm, blood islands appear as dense “patches” in a horse-shoe shaped area to each side of the neural plate and “in front of” (cranial to) the prochordal plate (buccopharyngeal membrane). These embryonic blood islands, in visceral, (splanchnic) mesoderm unite to form cords, and ultimately, a plexus of vessels. The central area of the resulting vascular plexus (“in front of” and lateral to the prochordal plate) represents the cardiogenic area where heart primordia, endocardial tubes, will form. Caudal to this region, paired dorsal aortae will form. 04. A multi-dimensional view of the embryo with amion removed. Transversely cut region shows the yolk sac below the developing embryo, and relationship of the developing blood islands to presumptive embryonic vessels including dorsal aortae. Yolk sac blood islands Recall that the space between somatic and visceral (splanchnic) lateral plate mesoderm is the embryonic coelomic cavity (presumptive pericardial, pleural and peritoneal cavities). 05. The portion of the coelomic cavity “in front of” and lateral to the prochordal plate (buccopharyngeal membrane) will become the pericardial cavity. Clusters of blood islands, formed from splanchnic (visceral) mesodermal mesenchyme, make up cardiogenic tissue that later will give rise to paired endocardial tubes. Yolk Sac 06. A parasagittal section depicts a section through one of the two developing endocardial tubes located in the floor (visceral mesoderm) of the primitive pericardial cavity. Note the position of the presumptive pericardial cavity and recall that cephalization ( growth in a cephalic direction) has caused “lifting” of the expanded neural plate (neural fold) so that it partially overlies the prochordal plate (buccopharyngeal membrane). 07. More rapid growth in a cephalic direction results in expansion of neural structures and flexion of the head region. Flexion creates a “pocket” beneath the head fold, the primitive mouth, or stomodeum, and causes ventral displacement of the prochordal plate, now more appropriately called buccopharyngeal membrane, since it is no longer “in front of” the notochord. Rotation (180 degrees) of the paired endocardial tubes, and pericardial cavity ultimately brings them into a position ventral to the developing foregut and caudal to the buccopharyngeal membrane. Vitelline veins, and later umbilical veins, form from blood islands in the yolk sac mesoderm and establish connections with the endocardial tubes at the venous end of the developing heart. Paired endocardial tubes are continuous with paired dorsal aortae, and with flexion, a pair of aortic arches is created at the arterial end of the developing heart. At the completion of heart rotation, the pericardial cavity has become ventral to the endocardial tubes and the transverse septum is positioned at the venous end of forming heart. 08. Ventral view. Lateral body folding brings paired endocardial tubes together in the ventral midline as the embryo becomes more tubular in shape. Fusion of endocardial tubes proceeds in a cephalic to caudal sequence. Fused endocardial tubes will make up the lining of the definitive heart. Later, lateral plate visceral (splanchnic) mesoderm gives rise to myocardial and epicardial layers. It should be noted that future heart chambers (stippled slashed, and cross-hatched in this illustration) are not all contained in the pericardial cavity. Initially, only the bulbus cordis (stippled) and ventricle (upper slashed) are located within the pericardial cavity. 09. The transverse section through a nearly tubular-shaped embryo (early during the fourth week) shows the relationships of the foregut, pericardial cavity and fusing endocardial tubes. Formation of the myoepicardial components is in progress at this time. The forming heart spontaneously begins to beat by 22 days. (A mesentery, the dorsal mesocardium, temporarily suspends the fused endocardial (cardiac) tube; this later ruptures and forms the transverse and oblique coronary sinuses of the pericardial sac.) 10. A slightly caudal transverse cut through the same embryo (as in #09) shows portions of the endocardial tubes that have not yet met in the ventral midline (have not fused). Lateral folding brings endocardial tubes together as the gut forms by the apposition and fusion of yolk sac (roof) endoderm. 11. Differential growth and expansion forms four heart chambers, in linear sequence and marked by grooves ( sulci). From cranial to caudal, the primitive heart chambers are: Bulbus cordis (with conus cordis) Ventricle Paired (left and right) atria Paired (left and right) sinus venosus Originally, the bulbus cordis and primitive ventricle are the only entire chambers that occupy the pericardial cavity. The arterial end of the primitive heart is held in place by the aortic arches. The venous end is also “fixed”, since paired atria and sinus horns are temporarily embedded in the mesodermal mesenchyme of the transverse septum. Mesenchyme consists ofundifferentiated, multipotential cells.) 12. The bulbus cordis delivers blood to the truncus arteriosus, which is continuous with paired aortic arches. These, in turn, are continuous with paired dorsal aortae. Later the truncus arteriosus gives rise to an aortic sac from which additional aortic arches arise, surround the foregut (pharynx), and join the paired dorsal aortae (see below). 13. The cardiac tube grows more rapidly than its surrounding pericardial cavity. This precocious growth gradually pulls the paired chambers into the pericardial cavity and causes the formation of a bulboventricular loop. Paired atrial components are moved together and fuse to form a single-chambered atrium. 14. Elongation of the cardiac tube causes it to bend and form a bulboventricular loop. At first the loop is “U”-shaped and directed anteriorly and to the right. The atrial dilation is directed posteriorly and to the left. 15. With continued growth the cardiac tube becomes “S”-shaped and the bulboventricular portion presents an even greater convexity (anterior and to the right). The atrium is moved posteriorly and toward the head. Paired sinus horns become partially fused, forming a transverse region of the sinus venosus and right and left sinus horns. 16. Growth and expansion accentuates the bulboventricular loop and forms a deep bulboventricular sulcus (groove). The internal ridge resulting from this external sulcus marks the site of future separation of definitive ventricles (see below). In the posterior view, illustrated here on the right, growth and expansion have also created a more transversely oriented atrial dilation that has been pulled posteriorly and cephalically. 17. In this illustration, the transversely oriented atrial dilation (that has been pulled posteriorly and cephalically) can be seen from a ventral view as only small atrial portions on each side of the conus cordis. The distal portion of the bulbus cordis is called the conus cordis. The conus cordis and the truncus arteriosus lie obliquely across the atrium to create a depression (like an arm indenting a pillow) that marks the future point of division of the atrium into right and left chambers. The transverse portion of the sinus venosus is moved posteriorly and cephalically. It joins the atrium at the sinu-atrial opening and is joined by the right and left sinus horns. The sinus horns receive, from medial to lateral, vitelline veins from the yolk sac, umbilical veins from the body stalk and fetal placenta, and common cardinal veins that receive the major veins of the body: precardinal (anterior cardinal) and postcardinal (posterior cardinal) that drain cranial and caudal portions of the embryo. (see below). 18. Hemodynamic changes in the venous channels result in most of the blood being carried through the right sinus horn. Because of the relationship of veins with the forming heart they are considered here, using dorsal (posterior) views. On the right: Right precardinal and right common cardinal veins form the superior vena cava. Right vitelline vein (proximal portion) forms the proximal portion of the inferior vena cava. Right horn of sinus venosus is incorporated into the right atrium (forms smooth portion, the sinus venarum). On the left: The proximal portion of the left sinus horn and the transverse portion of the sinus venosus form the coronary sinus. The distal portion of the left sinus horn, and possibly a portion of the common cardinal vein, form the oblique vein of the left atrium (of Marshall). Left vitelline, umbilical and cardinal veins are obliterated. Primitive pulmonary veins are incorporated into the left atrium and form four pulmonary veins. POSTERIOR VIEWS 19. Internal features of the forming heart are observed in coronal (frontal) and sagittal sections. Primitive trabeculae develop in both the proximal portion of the bulbus cordis and the primitive ventricle. The broad junction between these two chambers is the primary interventricular foramen. Superiorly this junction (foramen) is marked by the bulboventricular ridge. The primary interventricular septum is created, by differential growth and expansion of both bulbus cordis and primitive ventricle, as a progressively elongating inferior thickening. The proximal portion of the bulbus cordis later will form the right ventricle, and the primitive ventricle will become the left ventricle. The atrioventricular canal or foramen is a broad transversely oriented opening between the atrium and both the future right ventricle (proximal bulbus cordis) and future left ventricle (primitive ventricle). Septation of the heart into right and left sides occurs from the middle of the 4th to the end of the 5th week. The atrioventricular canal will become partitioned by mesenchymal masses called endocardial cushions that divide the canal into right and left atrioventricular canals. Lateral endocardial cushions contribute to the atrioventricular valves. (Failure of fusion of endocardial cushions results in a single A-V canal with abnormal valve leaflets, and defective atrial and ventricular septation -uncommon.) (Obliteration of the right A-V canal is called tricuspid atresia. It is caused by overgrowth of endocardial cushions or fusion of valves. It may be associated with patent foramen ovale (see below), ventricular septal defect, underdeveloped right ventricle and hypertrophied left ventricle). 20. Atrial septation begins with the formation of the septum primum (weeks 5 to 6). When the atrium is deeply grooved by the conus cordis (distal portion of the bulbus cordis) and truncus arteriosus, the external depression is reflected internally as an initially small sickle-shaped crest, the early septum primum. It extends from the atrial roof into the atrial lumen. 21. With growth, the sickle- shaped septum primum extends toward the endocardial cushion; it increases in size and thus progressively descends (like a shade being drawn down). The opening beneath the free edge of theseptum primum is called the ostium primum. It becomes smaller as the septum primum approaches the endocardial cushion. The ostium primum ultimately will close, as the septum primum fuses with the endocardial cushion (failure of this fusion is rare). Prior to closure, perforations appear in the superior portion of the septum primum. 22. Perforations in the septum primum coalesce to form a single opening, the ostium secundum. Soon after the formation of the ostium secundum, a flange or fold, the septum secundum, appears to the right of the septum primum, between it and the valve of the sinuatrial opening. 23. The septum secundum is also sickle-shaped; It progressively enlarges and descends toward the endocardial cushion. It forms an incomplete partition and the opening beneath its free edge is the foramen ovale. For a time, blood flows from the right atrium through the foramen ovale, between septum secundum and septum primum, through ostium secundum and into the left atrium. Ultimately, the tissue superior to the ostium secundum breaks down so that the septum primum then acts as a flap valve that covers the foramen ovale. Septum primum and secundum together make up the interatrial septum. It serves as a one-way valve, allowing blood to flow from the right atrium to the left atrium during fetal life. After birth, flow of blood from the umbilical vein ceases and a large volumn of blood from the lungs is returned to the left atrium. Pressure in the left atrium is thus increased and the flap valve in the interatrial septum is functionally closed. Anatomical closure usually follows shortly thereafter. Atrial septal defects may be due to defective endocardial cushions, excessive resorption of septum primum (common), or inadequate development of septum secundum (uncommon). Failure of interatrial septum formation results in a common (single) atrium, a condition referred to as cor triloculare biventriculare (heart with 3 chambers and 2 ventricles – very rare!). Premature closure of the foramen ovale results in massive hypertrophy of both the right atrium and the right ventricle. 24.The right ventricle develops from the proximal (trabeculated) part of the bulbus cordis. The left ventricle develops from the primitive ventricle of the early tubular heart. The opening between the the bulbus cordis and the primitive ventricle is the primary interventricular foramen. Superiorly (and posteriorly), the primary interventricular foramen is bordered by the bulboventricular ridge. Inferiorly, it is bordered by the primary interventricular septum. It should be noted that these two bounding structures are, in fact, continuous with each other (see below). 25. The muscular interventricular septum, due to growth and expansion of both the bulbus cordis (forming right ventricle) and the primitive ventricle (forming left ventricle), is increased in height. As the interventricular septum approaches, but does not yet meet the endocardial cushion, it forms the muscular interventricular septum. Separation of the forming ventricles will not occur until the membranous interventricular septum is formed. The fusion of the muscular interventricular septum with endocardial cushions and conus cordis ridges will complete the ventricular separation . A pair of spirally oriented, opposing conotruncal swellings ( ridges) appear in the wall of the conus cordis and truncus arteriosus (see below). 26. With growth, there is fusion of the pair of spirally oriented, opposing conotruncal ridges to form the aorticopulmonary septum. This septum divides both conus cordis and truncus arteriosus into an aortic trunk and a pulmonary trunk. Fusion of the muscular interventricular septum with both the endocardial cushions and fused ridges of the conus cordis (proximal aorticopulmonary septum) forms the membranous interventricular septum (septum membranaceum) and completes the separation of the ventricles. The membranous interventricular septum separates the apical regions (outflow tracts) of the right and left ventricles. Membranous I.V. Septal defects result from failure of fusion of the endocardial cushion and conus ridges with the muscular interventricular septum (common). Persistent Truncus Arteriosus is due to failure of truncal components of the conotruncal ridges to form. Transposition of Great Vessels: failure of conotruncal ridges to spiral. Tetralogy of Fallot: unequal partitioning of conotruncal (bulbotruncal) region and failure of closure by the membranous interventricular septum results in: 1.pulmonary stenosus, 2.interventricular septal defect, 3. overriding aorta (overrides both ventricles), 4. right ventricular hypertrophy (common) Neural crest cells contribute to the conotruncal ridges. Defective septation into pulmonary and aortic channels is frequently observed in company with craniofacial malformations of neural crest origin. Many thanks to Dr. Caroline Goode Jackson, Ph.D., for diagrams used in this syllabi and in lectures!! EMBRYOLOGY - SAMPLE QUESTIONS – Development of the HEART, Dr. Knapp 1. Which of the following are sites of vessel formation? A. Extraembryonic mesoderm of yolk sac. B. Cardiogenic tissue C. Visceral (splanchnic) mesoderm of embryo D. Embryonic blood islands E. All of the above 2. Which of the following is NOT considered to be a component of the early heart tube? A. Bulbus cordis D. Primitive ventricle B. Dorsal aorta E. Primitive atrium C. Sinus venosus 3. The truncus arteriosus of the primitive heart: A. Is later partitioned by the aorticopulmonary septum B. Is not a definitive structure in normal adults C. Gives rise to the aorta and the pulmonary trunk D. Is synonymous with the ductus arteriosus E. “A”, “B” and “C” 4. The U-shaped bulboventricular loop of the primitive heart forms as a result of the: A. Growth of the bulbus cordis and primitive ventricle B. Fixation of the arterial end of the heart C. Fixation of the venous end of the heart D. All of the above 5. The fetal right atrium is mainly derived from the: A. Primitive pulmonary veins C. Primitive atrium B. Right pulmonary vein D. Sinus venosus 6. Closure of the foramen primum results from fusion of the: A. Septum primum and the septum secundum B. Septum secundum and the septum spurium C. Septum primum and the endocardial cushion D. Septum secundum and the endocardial cushion E. Septum primum and the right sinoatrial valve 7. The distal portion of the bulbus cordis, called the conus cordis, of the embryonic heart: A. Forms the outflow tract (at base of aortic trunk) of the left ventricle. B. Forms the outflow tract (at base of pulmonary trunk) of the right ventricle. C. Becomes subdivided by the proximal portion of the aorticopulmonary septum D. All of the above 8. Early during the separation of heart chambers the atrioventricular foramen is subdivided by the: A. Septum primum D. Muscular interventricular septum B. Septum secundum E. Bulboventricular ridge C. Endocardial cushions ANSWERS: 1 = E; 2 = B; 3 = E; 4 = D; 5 = D; 6 = C; 7 = D; 8 = C