* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Notes on kinetic and potential energy
Photoelectric effect wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Marcus theory wikipedia , lookup
Atomic theory wikipedia , lookup
Energy harvesting wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Transition state theory wikipedia , lookup
Molecular dynamics wikipedia , lookup
Internal energy wikipedia , lookup
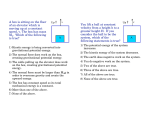
Notes on kinetic and potential energy Dalton’s atomic theory says that atoms are not destroyed or created in chemical reactions, just rearranged. We can be even more explicit and say that the number of atoms, and the mass of each atom, are not changed in a chemical reaction. Thus there is overall conservation of mass during chemical reactions. This conservation concept is rather easy to grasp and was verified experimentally in the 18th century. This was before Dalton, and thus was a fact that Dalton had to account for as he constructed his atomic theory. Another quantity that is intimately involved in chemical reactions is energy. The evidence for this is quite simply that chemical reactions produce a change in temperature, or useful work (such as burning gasoline to move a car). Many reactions are exothermic, meaning heat is produced. Others are endothermic, meaning heat is absorbed. These observations might lead you to believe, mistakenly, that chemical reactions produce energy or destroy energy. After all: In an exothermic reaction, the heat seems to come from nowhere. In an endothermic reaction, the absorbed heat seems to disappear. Appearances are deceiving! The energy does not “come from nowhere” or “disappear”. Rather, the heat comes from, or is converted to, another form of energy called potential energy. Thus, chemical reactions involve the transformation of energy from one form to another – not the creation or destruction of energy. In a chemical reaction, the total amount of energy is constant. In other words, energy, like mass, is conserved. This statement assumes that there is no exchange of energy between the “system” and the “surroundings”, or that we are counting the energy of both the system and surroundings, i.e., the whole universe. Yes, chemists really talk about the energy of the universe. So, what exactly is potential energy, anyway? It is the energy due to the attraction or repulsion of two objects. Another definition says it is the energy due to the relative position of two objects. Whatever words you use, potential energy depends on these four features: 1. There are two (or more) objects involved. 2. The objects have to attract or repel one another. 3. The amount of potential energy depends on the distance between the objects. 4. The amount of potential energy also depends on the mass (gravitational potential energy) or charge (electrical potential energy). One very familiar form of potential energy is gravitational potential energy. Gravitational interactions are always attractions, never repulsions. What's attracting the bike rider in this picture as he heads down the hill? As the bike and rider coast down the hill, their potential energy decreases. At the same time, their kinetic energy increases (by the same amount) because they are going faster and faster. Kinetic energy is energy due to movement of an object. The faster the object goes, the greater its kinetic energy. At the top of the hill, standing still, the rider has zero kinetic energy. As he coasts down the hill, he gains kinetic energy as he accelerates. The total energy stays constant (we’re ignoring friction, wind resistance, and the contributions of the bicyclist pedaling, for simplicity). In addition to its dependence on speed, KE is also affected by the mass of the object. A ping-pong ball and a baseball moving at the same speed don't have the same KE. You can guess which one has more – which would be more likely to break a window??? We can summarize the gravitational system as a model of an attractive interaction: 1. The potential energy is greatest when the objects are at maximum separation. 2. The potential energy decreases as the objects move closer to one another. Energy in chemical systems Gravity, although it is an instructive example of a potential energy system, plays no role in chemistry because the masses of atoms are so tiny. In contrast, electrical potential energy plays a huge role in chemistry. As we will be discussing before long, atoms are composed of three kinds of fundamental particles: protons (positively charged), electrons (negatively) and neutrons (uncharged). Like charges repel, whereas opposite charges attract. As was true for gravitational PE, the potential energy of an proton/electron pair is highest when the electron is far from the proton. On the other hand, an electron-electron or proton-proton pair have their highest PE when the particles are close together because they repel one another. An atom is a rat’s nest of interactions between charged particles – proton/proton, proton/electron, and electron/electron. The overall electrical PE is the sum of all the pairwise interactions. In molecules, there are further attractions and repulsions between the electrons and protons of one atom and those of an adjacent atom. If the attractive interactions were not there, compounds could not exist! We refer to these interactions, both those within atoms and those between atoms in a molecule, as chemical energy,or Ech. In addition, there are attractions between molecules. If these didn't exist, we couldn't have liquids or solids! Although this potential energy is fundamentally no different in nature from the chemical energy in atoms and molecules, for convenience we will track it separately and give it the name phase energy (Eph). Finally, the molecules in any chemical system are in motion and therefore possess kinetic (thermal) energy, which we will designate Eth. With these three categories, we have a convenient and reasonably accurate way of categorizing what happens to energy during various transformations. Specifically, * If we rearrange the atoms to make new chemicals, we are changing E ch * If we are changing the phase of a substance, we are changing E ph * If we are changing the temperature of a substance, we are changing E th Modes of energy transfer In the bike rider example, the rider's potential energy became kinetic energy as he coasted down the hill, but it was still his energy. Commonly, in chemical situations, we don't just transform the energy, we transfer it to another object. This can happen in several ways. 1. Work is energy is transferred between objects as they exert forces on one another. Imagine one billiard ball that strikes another one. The first one stops moving and the struck ball takes off. The first ball has performed work on the second ball. In the process, the KE of the first ball has decreased and the KE of the second ball has increased. When gasoline combustion is used to move your car, work is performed when the hot combustion gases push on the pistons in the motor. 2. Heat is the transfer of energy by the collisions of countless molecules. Energy is always transferred from the “hotter” object (one in which the molecules have greater E th) to a colder one (one in which the molecules have lower Eth). 3. Radiation is the transfer of energy by the absorption or emission of photons (particles of light). A light bulb filament can be heated to the point that it glows; this is the emission of photons that carry energy away from the filament. You can be warmed by light from the sun as the photons transfer energy to you. We can use a graphical organizer to display both the changes in stored energy and the mechanism of energy transfer in a given scenario. This is meant to be a qualitative rather than a quantitative picture. Finish these problems for homework 1. A cup of hot coffee cools as it sits on the table. 2. A can of cold soda warms as it is left on the counter. 3. A tray of water (20 ˚C) is placed in the freezer and turns into ice cubes (- 8 ˚C) 4. Where does the energy that leaves the system in #3 go? How does this energy transfer affect the room temperature in the kitchen? Do you have any experience that supports your answer? 5. You boil a kettle of water on the stove. 6. Natural gas is combusted in a Bunsen burner.